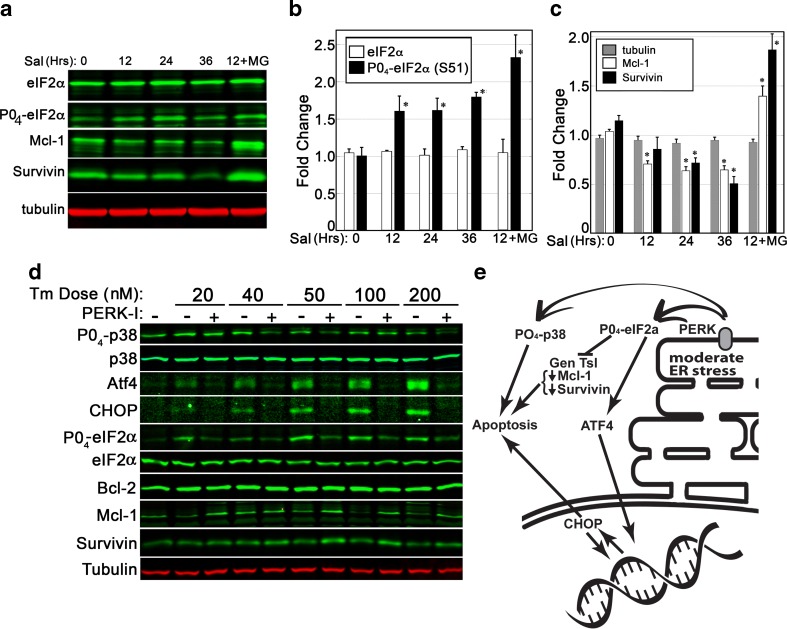
Fig. 7.
PERK activity plays a central role in pro-apoptotic cellular changes during ER stress. a–c A 36-h time course of salubrinal exposure (10 μM) was used to assess if eIF2α phosphorylation was sufficient to cause a decline in anti-apoptotic proteins. Quantitative analysis of BHK21 cell lysates revealed salubrinal, an inhibitor of the Gadd34 phosphatase, to cause a significant accumulation of phosphorylated eIF2α within 12 h. Levels of Mcl-1 and survivin proteins declined gradually over time. Inclusion of a proteasome inhibitor (MG132, 5 mM) was sufficient to arrest the decline in Mcl-1 and survivin. d BHK21 cells were exposed to varying doses of Tm over a period of 24 h and then analyzed via immunoblot. Inhibition of PERK was sufficient to suppress phosphorylation of p38 and eIF2α, decreases in survivin and Mcl-1, and induced expression of ATF4 and CHOP. Blots shown are representative of two replicated experiments. e A summary of PERK impacts upon stress signaling and apoptosis. Statistical assessment for b/c was by ANOVA with a Tukey’s all pairs post hoc text comparing samples at each time point to control (p < 0.5)