Table 3.
Reactions with various aryl bromides.
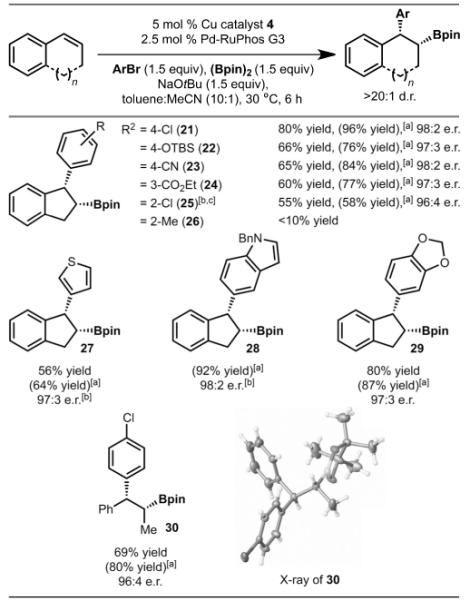
|
Yield reported as the average of at least two experiments. Diastereomeric ratio (d.r.) was determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture. Enantiomeric ratio (e.r.) was determined by HPLC analysis (with a chiral column) of the purified reaction mixture.
Yield determined by 1H NMR spectroscopic analysis of the unpurified reaction mixture with an internal standard.
5 mol% copper catalyst (4) was added in two portions, (see the Supporting Information for details).
Generated in 18:1 d.r.
