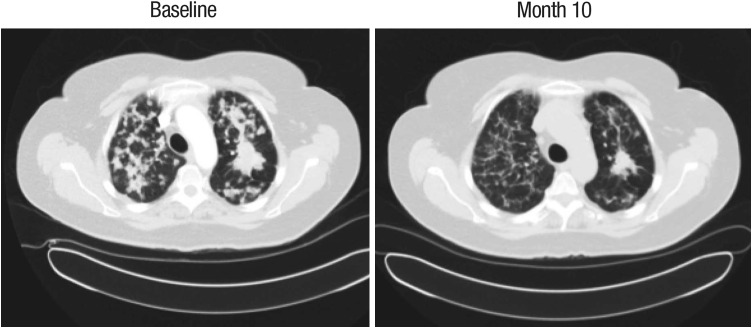
Fig. 3.
Computed tomography images showing an unconfirmed partial response in a patient with NSCLC who received afatinib 30 mg, paclitaxel 80 mg/m2, and bevacizumab 5 mg/kg. This patient received afatinib for 629 days. A partial response was reported twice in this patient during the study; however, these are considered to be unconfirmed partial responses because, at the consecutive tumor assessments, the percentage change in tumor lesions did not meet RECIST v1.0 criteria for partial response