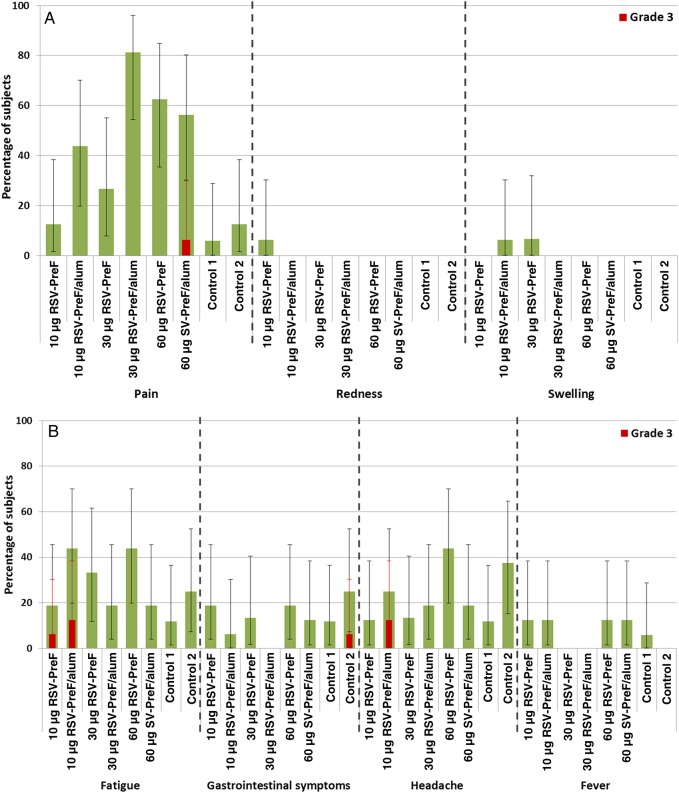
Figure 2.
Solicited injection site adverse events (A) and general adverse events (B) during the 7 days after vaccination—total vaccinated cohort. Grade 3 (severe) pain was defined as pain that is significant at rest and prevents normal everyday activities. Redness and/or swelling were considered present if the greatest surface diameter of each was >100 mm. A grade 3 (severe) systemic adverse event was defined as an event that prevents normal everyday activities. Saccharose NaCl served as the control. Error bars depict 95% confidence intervals. Abbreviation: RSV-PreF, respiratory syncytial virus engineered to preferentially maintain a prefusion conformation.