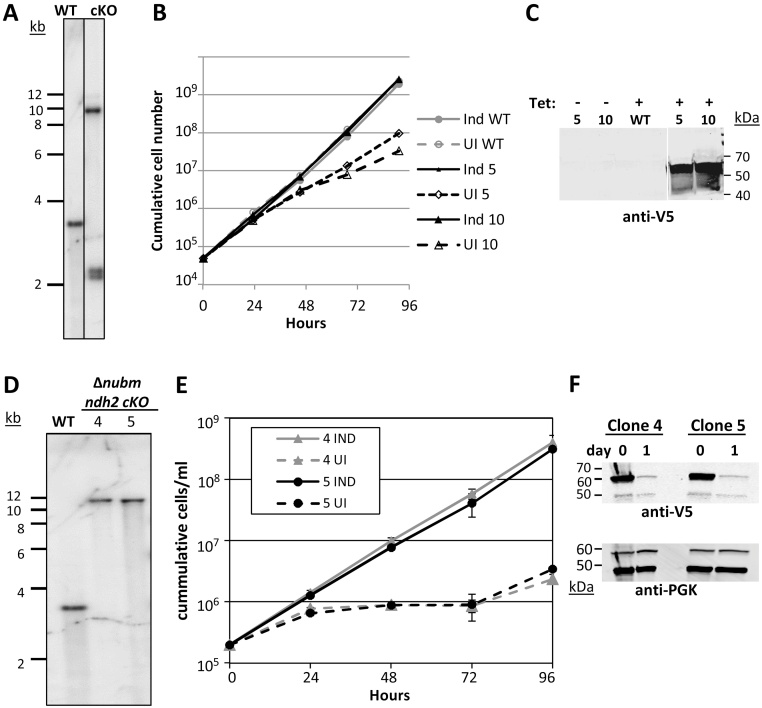
Fig. 1.
NDH2 is advantageous but not essential for growth of BF T. brucei in vitro. (A) Southern blot analysis of the NDH2 locus. Genomic DNA was isolated from the parental ‘wild type’ (WT) line and ndh2 cKO clone 10. Following digestion with SnaBI and probing with the NDH2 CDS, the expected band sizes are: WT, 3.6 kbp; cKO, >5.5, 2.4, and 2.3 kbp. As the largest fragment in the cKO cell line represents integration of the plasmid bearing the complementing gene into one of the rDNA loci, it is not possible to accurately predict the fragment size. The lanes are from the same gel and hybridization. (B) Cumulative growth curves of tetracycline (Tet)-treated (induced, Ind) and uninduced (UI) ndh2 cKO cells is compared to the parental WT. For uninduced (UI) cells Tet was removed from the medium at time point zero. Two clones (5 and 10) were analyzed. This experiment was performed in duplicate and 91% of the replicates on days 1–4 were within 15% of the mean. (C) Assessment by immunoblot of levels of ectopic, V5-tagged NDH2 in uninduced (-Tet) vs. induced cells (+Tet) on day 5. Gels (5 × 106 cell equivalents per lane) were transferred to nitrocellulose membranes, blocked and incubated with mouse anti-V5 monoclonal antibody at 0.5 μg/ml. Anti-V5 was detected by goat anti-mouse IgG-IRDye 800CW using a Li-Cor Odyssey system. NDH2-V5 has a predicted molecular weight of ∼60 kDa. Lanes are from the same scan of the western blot. (D) Southern analysis confirming the ndh2 cKO genotype in the Δnubm background. Genomic DNA digested with SnaBI was analyzed by Southern blot using a probe consisting of the NDH2 coding sequence. Expected sizes are: WT, 3.6 kbp; Δnubm ndh2 cKO clones 4 and 5, >5.6 kbp (the complementing copy is in one of the rDNA loci, so its exact size cannot be predicted). The NUBM knockout was confirmed in a previous study [12]. (E) Cumulative growth in the presence and absence of the inducer Tet of parasites with all endogenous alleles of NUBM and NDH2 deleted and harboring an inducible, V5-tagged copy of NDH2. For uninduced (UI) cells Tet was removed from the medium at time point zero. Two individual clones, 4 and 5, were analyzed. Error bars mark the standard deviation of the triplicate data points. For both clones, the calculated doubling times were ∼9.3 h in the presence of Tet and >100 h in the absence of Tet (days 1–3). (F) Western analysis of NDH2-V5 expression in the cKOs upon Tet withdrawal, in parallel with panel E. The same blot was re-probed with anti-phosphoglycerate kinase (PGK) as a loading control. The return to normal growth rates 3–4 days after Tet removal is most likely due to loss of repression of the ectopic NDH2 gene, as was seen in other experiments (see Fig. S2).