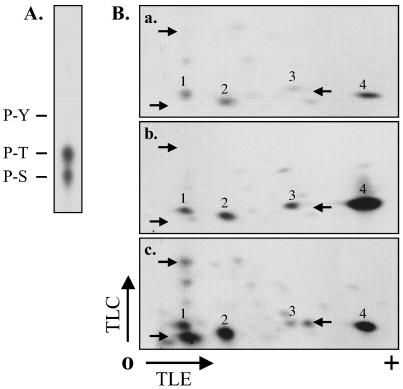
FIG. 8.
Phosphoamino acid analysis and peptide mapping of phosphorylated ROP4. (A) Phosphoamino acid analysis. Purified ROP4 from [32P]orthophosphate-labeled infected HFF cells was hydrolyzed with 6 N HCl and analyzed by TLC. 32P-labeled spots comigrating with ninhydrin-stained phosphoserine (P-S), phosphothreonine (P-T), and phosphotyrosine (P-Y) standards were detected by autoradiography. (B) Phosphopeptide mapping. ROP4 was immunoprecipitated with MAb C8.4 from parasite extracts labeled with [γ-32P]ATP (a), combined HFF and parasite extracts labeled with [γ-32P]ATP (b), or extracts prepared from [32P]orthophosphate-labeled infected HFF cells (c). Proteins were resolved by SDS-PAGE, transferred to Immobilon-P, and detected by autoradiography. In each case, the band corresponding to ROP4 was excised, digested with TPCK (tosylsulfonyl phenylalanyl chloromethyl ketone)-trypsin, and spotted onto the origin (O) of a cellulose TLC plate. Tryptic peptides were separated by electrophoresis in the first dimension and chromatography in the second. The labeling intensity of two of the phosphopeptides increased dramatically in the combined host cell-parasite extract compared to that in the extract of parasites alone (a and b, spots 3 and 4). Several additional phosphopeptides were detected in the infected HFF cells (c, arrows) that were not phosphorylated in cell extracts (a and b, arrows). TLE, thin-layer electrophoresis.