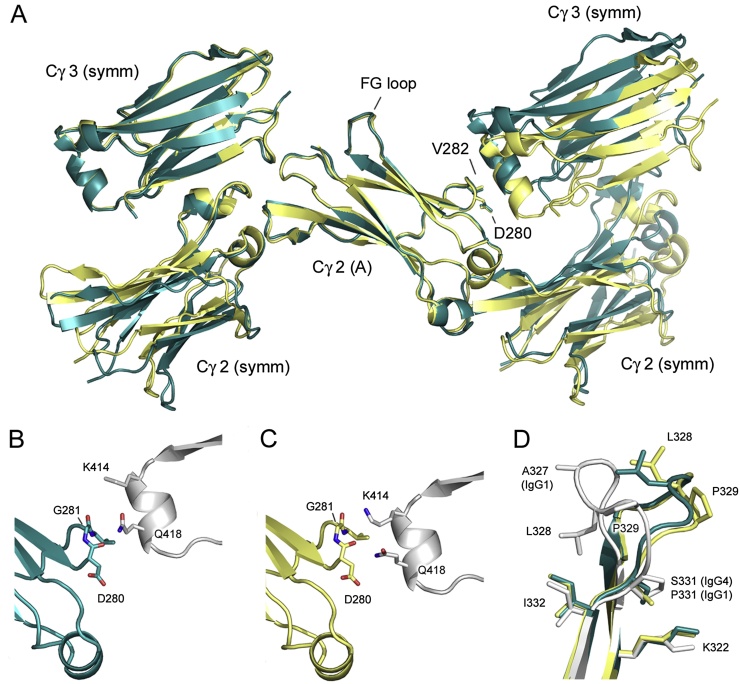
Fig. 3.
Crystal packing and Cγ2 domain FG loop conformation (chain A). (A) With the exception of residues Asp280-Val282, crystal packing interactions in the room temperature (RT) (blue) and cryogenic (yellow) (Davies et al., 2014b) IgG4-Fc structures, which do not involve the FG loop, are similar. (B) In the RT structure (blue) Gln418 from the Cγ3 domain of a symmetry-related molecule (gray) packs against residues Asp280, Gly281 and Val282. (C) In the cryogenic structure (yellow), Lys414 packs against Gly281, and Gln418 forms a hydrogen bond with the Asp280 carbonyl atom. (D) In chain A of the RT (blue) and cryogenic (yellow) structures, the Cγ2 FG loop from chain A adopts a different conformation to the conserved conformation found in IgG1 (gray) (Ferrara et al., 2011). Figures (A–C) were generated after superposition on the Cγ2 domain from chain A. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)