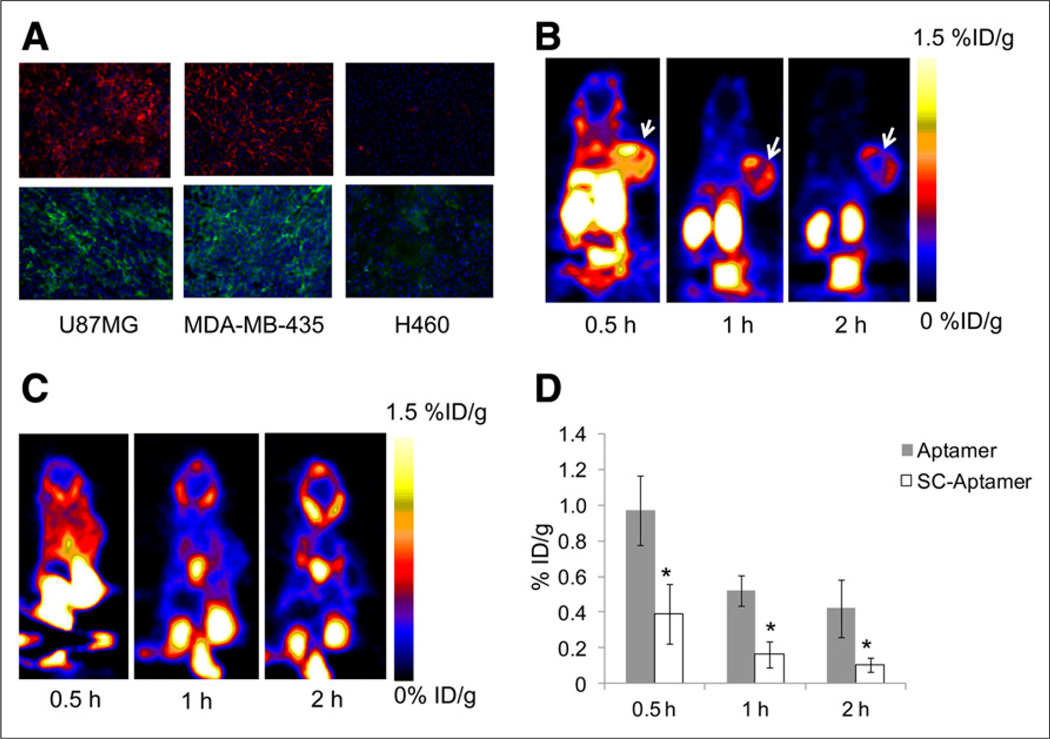
FIGURE 4.
(A) Immunofluorescence staining of tenascin-C in frozen section slides of tenascin-C–positive xenografts U87MG and MDA-MB-435 and tenascin-C–negative xenograft H460 using anti–tenascin-C antibody (upper) and FITC-labeled tenascin-C aptamer (lower). Magnification, ×200. (B) Representative coronal PET images of mice bearing subcutaneous U87MG xenograft injected with 18F-FB-tenascin-C aptamer at indicated time points. White arrow indicates tumor. (C) Representative coronal PET images of mice bearing subcutaneous H460 xenograft injected with 18F-FB-tenascin-C aptamer at 30 min and 1 and 2 h after injection. White arrow indicates site of tumor. (D) Comparison between 18F-FB-tenascin-C aptamer and 18F-FB-Sc aptamer in tumor uptake at different time points (0.5, 1, and 2 h after injection) (n = 4/group). *Significance between aptamer and Sc aptamer tumor uptake.