Figure 2. p21 is phosphorylated at S130 by Cdk1/cyclin B1.
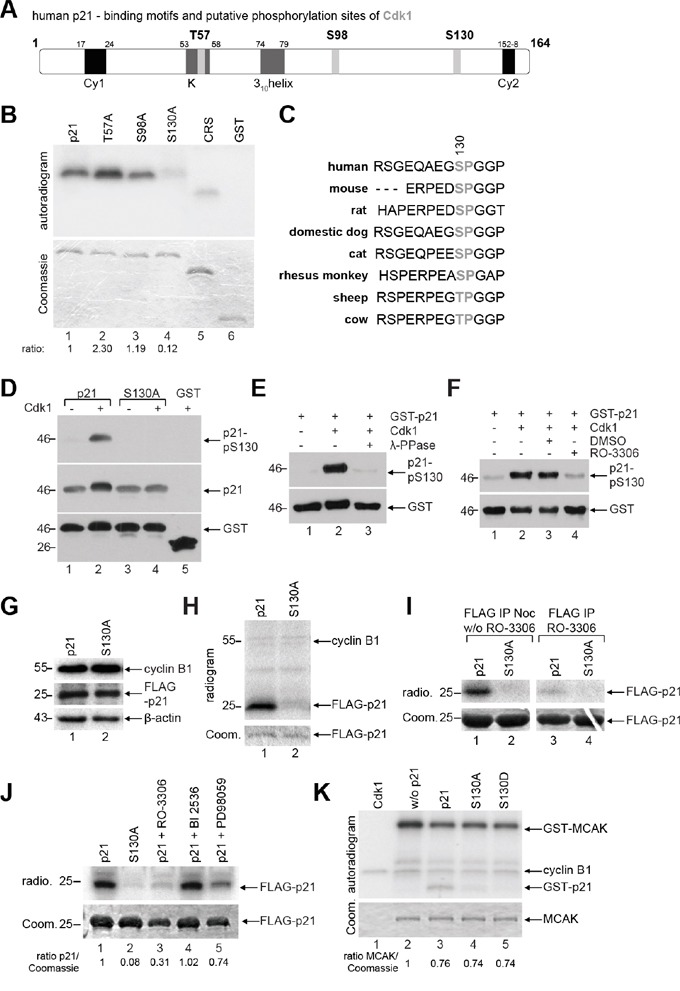
A. Schematic illustration of human p21 including its putative phosphorylation sites of Cdk1 with the minimal consensus motif pT/pS-P (light gray). Its two cyclin binding motifs Cy1 and Cy2 (black), the Cdk binding site K and the 310helix (dark gray) are also shown. B. In vitro kinase assay of human GST-tagged p21 and its mutants, whereby each threonine or serine was replaced by alanine. CRS (cytoplasm retention signal) of cyclin B1 and GST proteins were taken as positive and negative control, respectively. The same gel was stained with Coomassie as input control. The phosphorylation intensities, relative to the input, are shown, evaluated by using ImageJ (National Institutes of Health). C. Sequence alignment of p21 from different species and the S130 residue is highlighted in gray. D-F. Different in vitro studies were carried out with the generated phospho-antibody against p21-pS130. (D) GST-p21 and its mutant S130A were subjected to in vitro kinase assay with non-radioactive ATP and Cdk1 kinase and further analyzed by Western blot analysis with indicated antibodies. The same membrane was stained with GST antibody as loading control. (E) In vitro kinase assay was performed with GST-p21 as described in (D), in the presence of phosphatase (λ-PPase) and analyzed by Western blot analysis. The same membrane was stained with GST antibody as loading control. (F) In vitro kinase assay with GST-p21, in the presence of the Cdk1 inhibitor RO-3306, was performed, and analyzed by Western blot analysis. The same membrane was stained with GST antibody as loading control. G. HeLa cells transfected with FLAG-tagged p21 or its mutant S130A were synchronized with nocodazole (noc) for Western blot analyses with antibodies against cyclin B1 and FLAG tag as cell cycle and transfection efficiency control, respectively. β-actin served as loading control. H. Ex vivo kinase assay. The extracts from (G) were immunoprecipitated with FLAG® M2 beads for a kinase assay without further addition of Cdk1/cyclin B1. The results are presented by an autoradiogram (upper panel). The Coomassie (Coom.) staining served as loading control (lower panel). I. Same experimental setup as in (H), incubation time without (w/o) and with addition of the Cdk1 inhibitor RO-3306 (9 μM). The results are presented by an autoradiogram (radio., upper panel). The Coomassie (Coom.) staining served as loading control (lower panel). J. Ex vivo kinase assay by using different kinase inhibitors: Cdk1 inhibitor RO-3306 (9 μM), Plk1 inhibitor BI2536 (25 nM) and MAP kinase cascade inhibitor PD98059 (10 μM). Ratio of p21 normalized against the Coomassie loading control is indicated. K. In vitro kinase assay of Cdk1/cyclin B1 with a known substrate MCAK (the mitotic centromere-associated kinesin), in the presence of GST-tagged wild type p21 or its mutants. The Coomassie (Coom.) staining of MCAK served as loading control.
