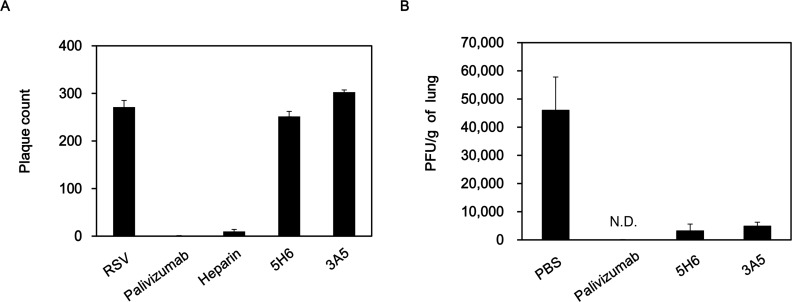
Fig 4. In vitro Neutralization and In vivo RSV clearance activity of 5H6 and 3A5.
(A) In vitro plaque reduction assay. Palivizumab, heparin, 5H6, and 3A5 were incubated with 200–300 PFU of RSV A2 for 1 hr at 37°C. Then, the mixture was added to HEp-2 cells for 1.5 hr, and the plaques were counted 5 days later. (B) Prophylactic treatment with mAb in mouse model. BALB/c mice were intramuscularly administered 100 μg of each mAb and challenged intranasally with 106 PFU of RSV A2 1 day later (n = 4 mice/group). On day 4 post-infection, lung homogenates were prepared, and lung viral titers were measured by plaque assay. The limit of detection was 94 PFU/gram of lung tissue. N.D., not detected.