Figure 1.
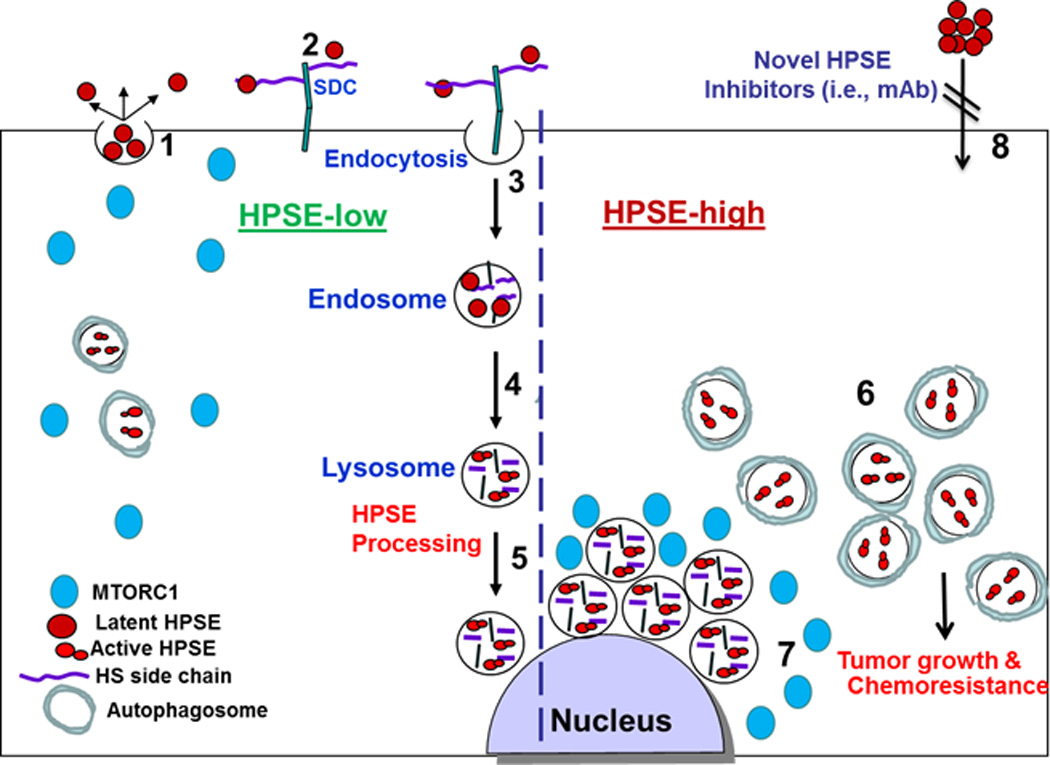
A schematic model of heparanase trafficking and function in autophagy. Once secreted (1), heparanase rapidly interacts with cell membrane HSPGs such as syndecans (SDC) (2), followed by a rapid endocytosis of the heparanase-HSPG complex (3). Conversion of endosomes to lysosomes (4) results in heparanase processing and activation (5). Typically, heparanase appears at perinuclear lysosomal vesicles (5). Lysosomal heparanase regulates the basal level of autophagy and resides within autophagosomes (HPSE-low). Cancer cells that exhibit high content of heparanase (HPSE-high) are endowed with increased autophagy (6) that promotes tumor growth and chemo resistance. Enhanced autophagy by heparanase is associated with reduced p70 S6-kinase phosphorylation levels and accumulation of mTOR1 at peri-nuclear areas (7) vs. more diffused distribution in control (HPSE-low) cells. Function of heparanase within the cell encourages the development of new class of inhibitors that will prevent heparanase uptake and lysosomal accumulation (8).
