Figure 2.
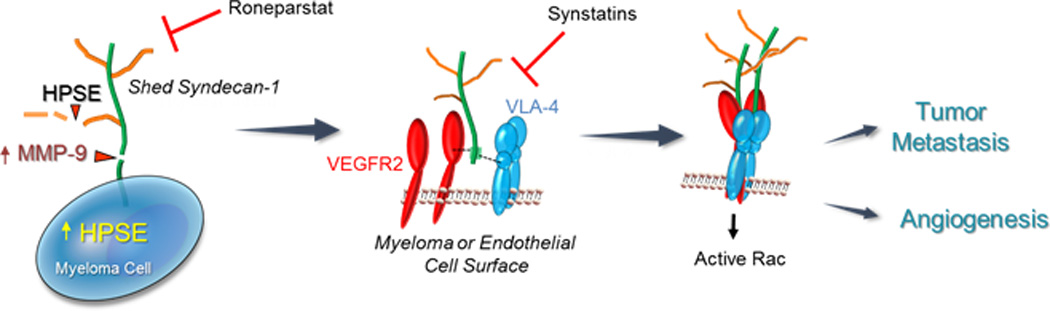
Heparanase activates a signaling mechanism that drives both tumor cell invasion and angiogenesis. (Left Panel) Myeloma cells express syndecan-1 on their cell surface composed of a core protein (green) and heparan sulfate chains (brown). Upregulation of heparanase (HPSE) expression by myeloma cells leads to trimming of syndecan-1 heparan sulfate chains, shortening their length and allowing increased access of proteases to the exposed syndecan-1 core protein. One such protease is MMP-9, a syndecan-1 sheddase whose expression is upregulated when heparanase is expressed by myeloma cells. MMP-9 cleaves the syndecan-1 core protein and the proteoglycan is shed from the cell surface. (Center Panel) Shedding of syndecan-1 exposes a cryptic domain within the juxtamembrane region of the core protein (green). Within this cryptic domain are amino acid sequences that bind to clustered VLA-4 (blue) and VEGFR2 (red) on the surface of myeloma cells or endothelial cells. (Right Panel) The coupling of VLA-4 and VEGFR2 receptors by shed syndecans activates VEGFR2 signaling that stimulates both cell invasion and endothelial tube formation. This signaling mechanism is inhibited by Roneparstat, a heparanase inhibitor that diminishes syndecan-1 shedding, or by synstatin peptides, peptide mimics of the syndecan-1 core protein that competitively inhibit binding of either VLA-4 or VEGFR-2 to shed syndecan-1.
