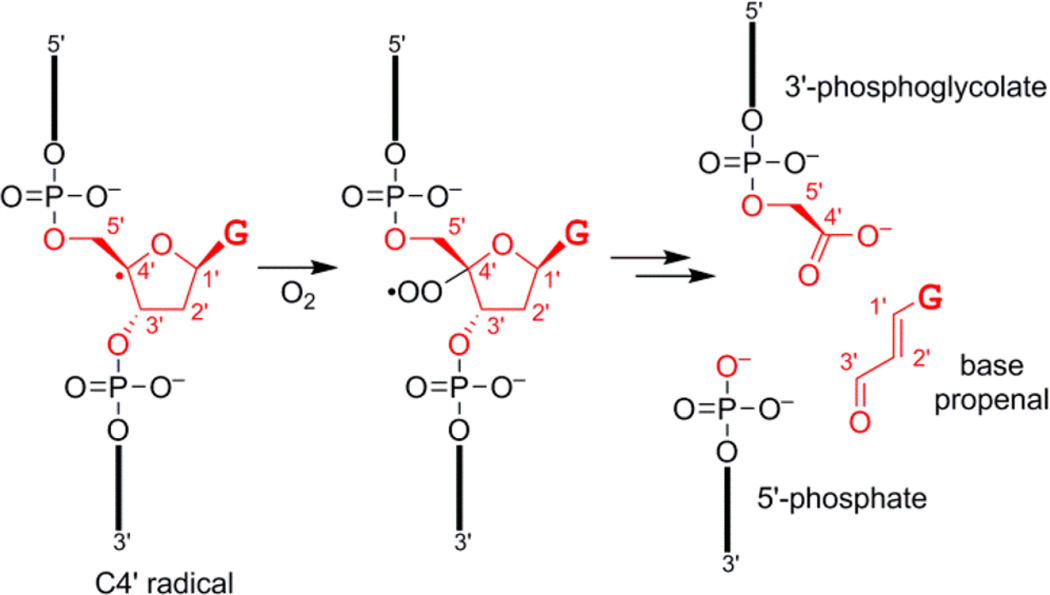
Figure 4.
DNA fragments formed upon reaction with the natural product bleomycin, shown starting with the C4′ radical intermediate and with excision of most atoms of a guanosine (G) nucleoside.14 Carbon atoms C1′-C5′ are marked on each structure. The 3′-phosphoglycolate, 5′-phosphate, and base propenal products are shown. The 3′-phosphoglycolate is a carboxylic acid and therefore capturable by the selection strategy of Figure 1.