Abstract
Background
Primary spontaneous pneumothorax (PSP) is a common benign disorder. However, unpredictable recurrence is a major concern for most patients. The aim of the present study was to assess the role of matrix metalloproteinase-2 (MMP-2) and MMP-9 in alveolar macrophages of patients with PSP and its relationship with recurrence.
Methods
Ninety-two patients who received needlescopic video-assisted thoracoscopic surgery (NVATS) wedge resection of lung with identifiable blebs for PSP were enrolled for the study. Immunohistochemistry was performed to evaluate the expression of MMP-2 and MMP-9 in lung tissues of patients with PSP. The result was correlated with clinicopathological variables and recurrence rates by the chi-square test. The value of MMP-2 and MMP-9 for overall recurrence was evaluated by univariate and multivariable Cox regression analyses.
Results
The MMP-2 and MMP-9 staining was predominantly observed in alveolar macrophages of patients with PSP. We found that MMP-2 (recurrence: P<0.001; smoking status: P=0.029) and MMP-9 (recurrence: P=0.001; smoking status: P=0.045) expression in PSP, especially male patients, was significantly correlated with recurrence and smoking status. In the multivariate analyses, MMP-2 [hazard ratio (HR) =2.83; 95% confidence interval (CI): 1.37–5.85, P=0.005) and MMP-9 (HR =2.25; 95% CI: 1.19–4.24, P=0.013) were statistically significant risk factors for overall recurrence in PSP patients.
Conclusions
High expression levels of MMP-2 and MMP-9 showed a positive correlation with recurrence in PSP patients. Further studies are required to test whether inhibition of MMP-2 and MMP-9 expression renders a promising approach for reducing the risk of PSP recurrence in the future.
Keywords: Pneumothorax, matrix metalloproteinase (MMP), recurrence, macrophage
Introduction
Primary spontaneous pneumothorax (PSP) is a common but serious disease with an incidence of 18–28/100,000/year for men and 1.2–6/100,000/year for women (1). PSP occurs without previous trauma or precipitating cause and arises in patients without clinically apparent underlying lung diseases. This disease mostly occurs in young adolescent males and is encountered in clinical practice. The incidence of ipsilateral recurrence of PSP is approximately 30%, with a range of 16–52% whilst the average rate of contralateral recurrence is around 15% after first episode of PSP (2-4).
PSP is caused by chronic and progressive destruction of subpleural alveolar structures at the lung due to oxidative stress, hypoxia, and chronic inflammation (5). PSP is associated with the degradation of elastic fibers and is mediated through infiltration of inflammatory cells including neutrophils and macrophages (6,7). The elastolysis is caused by an imbalance between the activities of proteases and antiproteases (8,9).
Matrix metalloproteinases (MMPs) constitute a family of zinc-dependent endopeptidases (10). Depending on substrate specificity, the MMP family can be classified into distinct subgroups: collagenases, gelatinases, stromelysins, elastases, and membrane-type MMPs (11). MMPs play a major role in the turnover of extracellular matrix that is needed in the processes of angiogenesis and tissue remodeling (12). PSP is caused by the rupture of blebs, which is related to the weakening of subpleural lung tissue. Recent reports have suggested that the gelatinases (MMP-2 and MMP-9) are critical for the clinical expression of some pulmonary diseases (10,13-15).
In PSP, macrophages play a crucial role in the chronic inflammation response (5). However, very limited information is available regarding MMP-2 and MMP-9 expression in alveolar macrophages of patients with PSP and its association with recurrence of PSP. In this study, we analyzed the expression levels of MMP-2 and MMP-9 in macrophages of both lung tissue specimens and surrounding normal tissue specimens by immunohistochemical (IHC) analysis. Their correlation with clinical characteristics were evaluated to determine whether expression levels of MMP-2 and MMP-9 could be clinical factors involved in recurrence of PSP.
Methods
Patient samples
We retrospectively reviewed the clinical records of 92 consecutive patients with PSP and who underwent surgery at the Division of Chest Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Taiwan, from January 2012 to December 2013. Inclusion criteria included PSP diagnosed by chest radiography and computed tomography (CT) scan as well as patient age ≤40 years. Patients with a secondary pneumothorax, as well as cases of traumatic or iatrogenic pneumothorax, clinical lung disease [i.e., chronic obstructive pulmonary disease (COPD), sarcoidosis, asthma, pneumonia, malignancy], pulmonary surgery (lobectomy, wedge resection of the lung) or lack of medical records were excluded. Indications for surgical treatment included recurrence, persistent air leak (for ≥5 days), or patient preference. Radiologic proof of ipsilateral or contralateral relapse of pneumothorax after first episode of PSP was defined as recurrence and their specimens with blebs and adjacent normal lung tissues were obtained while undergoing surgery. The patients who underwent surgery during their first admittance with PSP and had no recurrence during a 2-year follow-up period were defined as the non-recurrence group. The patients received regular postoperative follow-up in our out-patient clinic for 2 years. The patients were educated and advised to return to our hospital immediately in case of any discomfort or symptoms similar to those of pneumothorax. Chest radiography was the routine for the suspected cases and CT scan would be performed if needed. All resected specimens were processed for IHC staining. The age, gender, body mass index (BMI, kg/m2), smoking status, and side of pneumothorax were recorded. This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-20130268).
Surgical technique
The surgical technique for needlescopic video-assisted thoracoscopic surgery (NVATS) blebectomy and mechanical pleurodesis has been described in our previous publications (16-18).
IHC analysis
IHC staining for MMP-2 and MMP-9 was performed on a Bond-Max autostainer (Leica Microsystems, Bannockburn, IL, USA). Sections on microscopic slides from paraffin-embedded tissue blocks were deparaffinized at 72 °C and rehydrated in bond wash solution. Heat-induced antigen retrieval was carried out with Bond Epitope Retrieval Solution 2 for 20 minutes at 100 °C and peroxide block placement on the slides for 5 minutes at room temperature. Slides were then incubated with MMP-2 (1:200, Abgent, San Diego, California, USA) and MMP-9 (1:100, Abgent, San Diego, California, USA) antibodies for 30 minutes and post primary reagent for 8 minutes at room temperature. Antibody detection was carried out with the Bond Polymer placement and color development was performed with DAB (3,3'-diaminobenzidine tetrahydrochloride). Slides were counterstained with hematoxylin, followed by mounting of the slides before images of IHC-stained sections were captured by a Nikon Eclipse Ti microscope (Tokyo, Japan) and processed by Nikon NIS-Elements Version 4.30 software (Tokyo, Japan). Negative control with non-addition of the primary antibodies was conducted in parallel with the IHC procedure. The specificity of staining was evaluated in comparison with a negative control specimen.
Slide evaluation
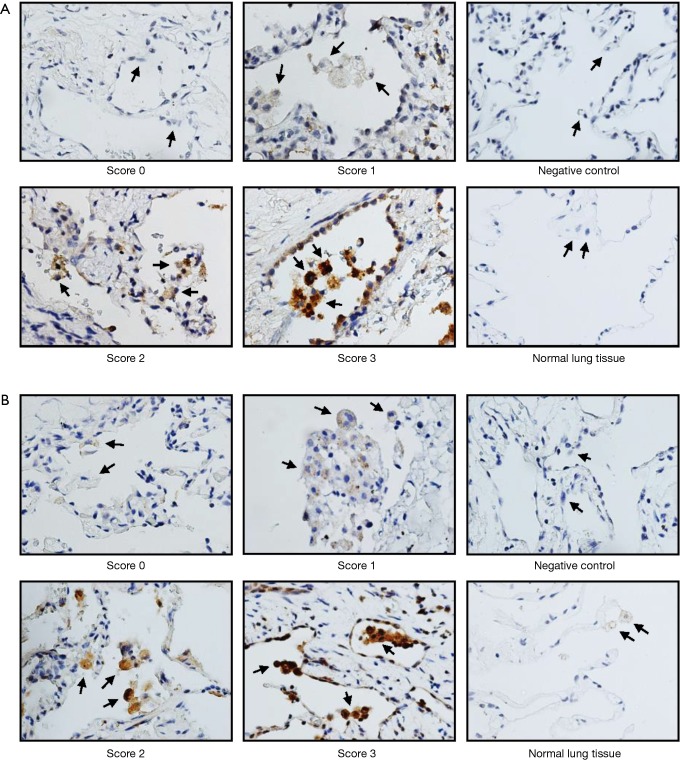
The results for MMP-2 and MMP-9 staining were determined by two independent experts under the same condition. Four visual fields were evaluated randomly and the staining intensity was stratified into quartiles. Staining intensity was scored from 0 to 3, denoting absent, weak, moderate, and strong staining respectively. The results for MMP staining were based on the staining intensity of positively stained cells. Scores 0, 1, and 2 were categorized as low expression, while score 3 was categorized as high expression for statistical analysis.
Statistical analysis
Comparisons between high-expression and low-expression groups of MMP-2 or MMP-9 with age, gender, BMI, recurrence, smoking status, and side involved were analyzed by the chi-square test. Hazard ratio (HR) and 95% confidence interval (CI) from univariate and multivariable Cox proportional hazards regression models were used to assess associations between overall recurrence and clinicopathological characteristics. All statistical analyses were performed using the SPSS 20.0 statistical package for PC (SPSS, Chicago, IL, USA). P values less than 0.05 were considered statistically significant.
Results
MMP-2 and MMP-9 expression levels in alveolar macrophages of PSP patients
Ninety-two patients, 79 males (85.9%) and 13 females (14.1%), with PSP were included in this study. The information for age, sex, BMI, smoking status, lesion location, and recurrence is summarized in Table 1. Forty-nine patients who presented with any recurrence (ipsilateral or contralateral) are shown in Table 2. In total, 79.6% (n=39) of the recurrence was in the ipsilateral recurrence group, while 20.4% (n=10) was in the contralateral recurrence group. Representative photographs for protein expression of MMP-2 and MMP-9 in alveolar macrophages are shown in Figure 1. The MMP-2 and MMP-9 staining was predominantly observed in lung tissues of PSP but not in the adjacent normal lung tissues.
Table 1. Clinical characteristics of patients with primary spontaneous pneumothorax.
| Characteristics | N=92 |
|---|---|
| Age (years) | 20.9±5.2 |
| Sex | |
| Male | 79 (85.9%) |
| Female | 13 (14.1%) |
| Height (cm) | 172.7±7.7 |
| Body weight (kg) | 56.6±9.2 |
| BMI (kg/m2) | 18.9±2.2 |
| Smoking | |
| Yes | 17 (18.5%) |
| No | 75 (81.5%) |
| Side involved | |
| Unilateral | 66 (71.7%) |
| Bilateral | 26 (28.3%) |
| Recurrence | |
| Yes | 49 (53.3%) |
| No | 43 (46.7%) |
Data are presented as means ± SD or number (%). BMI, body mass index; SD, standard deviation.
Table 2. Clinical characteristics of patients with recurrent primary spontaneous pneumothorax.
| Characteristics | N=49 |
|---|---|
| Age (years) | 19.9±4.7 |
| Sex | |
| Male | 42 (85.7%) |
| Female | 7 (14.3%) |
| Height (cm) | 172.2±7.5 |
| Body weight (kg) | 56.3±9.4 |
| BMI (kg/m2) | 18.9±2.2 |
| Smoking | |
| Yes | 6 (12.2%) |
| No | 43 (87.8%) |
| Side involved | |
| Unilateral | 35 (71.4%) |
| Bilateral | 14 (28.6%) |
| Recurrence type | |
| Ipsilateral recurrence | 39 (79.6%) |
| Contralateral recurrence | 10 (20.4%) |
Data are presented as means ± SD or number (%). BMI, body mass index; SD, standard deviation.
Figure 1.
IHC staining for MMP-2 (A) and MMP-9 (B) in alveolar macrophages of PSP. Staining was scored according to the staining intensity in four quantitative categories (score 0, absent staining; score 1, weak staining; score 2, moderate staining; and score 3, strong staining). The expressions of MMP-2 and MMP-9 in the matched adjacent normal lung tissues and negative control are also shown. The representative photographs are shown with 400× magnification. MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; IHC, immunohistochemical.
Correlation of MMP-2 and MMP-9 expression levels in PSP with clinicopathological characteristics
The expression levels of MMP-2 and MMP-9 in PSP were correlated with clinicopathological variables including age at diagnosis, sex, BMI, recurrence, smoking status, and side involved (Table 3). We found that high MMP-2 expression in pneumothorax was significantly correlated with increased recurrence risk (P<0.001) and smoking status (P=0.046), while increased MMP-9 expression was positively correlated with increased recurrence (P=0.001) (Table 3). The age, gender, BMI, and side involved in high-expression and low-expression MMP-2 and MMP-9 groups were not significantly different (Table 3). Further analysis in male patients revealed increased disease recurrence and smoking status in high MMP-2 groups (recurrence: P<0.001; smoking status: P=0.029) (Table 4). Similar results were found in MMP-9 groups (recurrence: P=0.001; smoking status: P=0.045) (Table 4).
Table 3. Clinical characteristics of pneumothorax patients with MMP-2 and MMP-9 expression.
| Variables | Item | Patient No. (%) | MMP-2 | MMP-9 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P valuea | Low | High | P valuea | ||||||||||
| No. | % | No. | % | No. | % | No. | % | ||||||||
| Age (years) | ≤20 | 52 (56.5) | 17 | 32.7 | 35 | 67.3 | 0.631 | 21 | 40.4 | 31 | 59.6 | 0.657 | |||
| >20 | 40 (43.5) | 15 | 37.5 | 25 | 62.5 | – | 18 | 45.0 | 22 | 55.0 | – | ||||
| Sex | Male | 79 (85.9) | 25 | 31.6 | 54 | 68.4 | 0.119 | 35 | 44.3 | 44 | 55.7 | 0.546 | |||
| Female | 13 (14.1) | 7 | 53.8 | 6 | 46.2 | – | 4 | 30.8 | 9 | 69.2 | – | ||||
| BMI (kg/m2) | <18 | 36 (39.1) | 11 | 30.6 | 25 | 69.4 | 0.495 | 14 | 38.9 | 22 | 61.1 | 0.586 | |||
| ≥18 | 56 (60.9) | 21 | 37.5 | 35 | 62.5 | – | 25 | 44.6 | 31 | 55.4 | – | ||||
| Recurrence | No | 43 (46.7) | 23 | 53.5 | 20 | 46.5 | <0.001 | 26 | 60.5 | 17 | 39.5 | 0.001 | |||
| Yes | 49 (53.3) | 9 | 18.4 | 40 | 81.6 | – | 13 | 26.5 | 36 | 73.5 | – | ||||
| Smoking status | No | 75 (81.5) | 30 | 40.0 | 45 | 60.0 | 0.046 | 35 | 46.7 | 40 | 53.3 | 0.106 | |||
| Yes | 17 (18.5) | 2 | 11.8 | 15 | 88.2 | – | 4 | 23.5 | 13 | 76.5 | – | ||||
| Side involved | Unilateral | 66 (71.7) | 21 | 31.8 | 45 | 68.2 | 0.342 | 30 | 45.5 | 36 | 54.5 | 0.343 | |||
| Bilateral | 26 (28.3) | 11 | 42.3 | 15 | 57.7 | – | 9 | 34.6 | 17 | 65.4 | – | ||||
a, the P value was calculated by the chi-square test. MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; BMI, body mass index.
Table 4. Clinical characteristics of male pneumothorax patients with MMP-2 and MMP-9 expression.
| Variables | Item | Patient No. (%) | MMP-2 | MMP-9 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P valuea | Low | High | P valuea | ||||||||||
| No. | % | No. | % | No. | % | No. | % | ||||||||
| Age (years) | ≤20 | 48 (60.8) | 14 | 29.2 | 34 | 70.8 | 0.556 | 21 | 43.8 | 27 | 56.2 | 0.902 | |||
| >20 | 31 (39.2) | 11 | 35.5 | 20 | 64.5 | – | 14 | 45.2 | 17 | 54.8 | – | ||||
| BMI (kg/m2) | <18 | 31 (39.2) | 9 | 29.0 | 22 | 71.0 | 0.688 | 13 | 41.9 | 18 | 58.1 | 0.733 | |||
| ≥18 | 48 (60.8) | 16 | 33.3 | 32 | 66.7 | – | 22 | 45.8 | 26 | 54.2 | – | ||||
| Recurrence | No | 37 (46.8) | 20 | 54.1 | 17 | 45.9 | <0.001 | 24 | 64.9 | 13 | 35.1 | 0.001 | |||
| Yes | 42 (53.2) | 5 | 11.9 | 37 | 88.1 | – | 11 | 26.2 | 31 | 73.8 | – | ||||
| Smoking status | No | 64 (81.0) | 24 | 37.5 | 40 | 62.5 | 0.029 | 32 | 50.0 | 32 | 50.0 | 0.045 | |||
| Yes | 15 (19.0) | 1 | 6.7 | 14 | 93.3 | – | 3 | 20.0 | 12 | 80.0 | – | ||||
| Side involved | Unilateral | 55 (69.6) | 16 | 29.1 | 39 | 70.9 | 0.460 | 26 | 47.3 | 29 | 52.7 | 0.421 | |||
| Bilateral | 24 (30.4) | 9 | 37.5 | 15 | 62.5 | – | 9 | 37.5 | 15 | 62.5 | – | ||||
a, the P value was calculated by the chi-square test. MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9; BMI, body mass index.
Association between expression levels of MMP-2 and MMP-9 in PSP tissues and overall recurrence in patients
To further investigate the association between MMP-2 and MMP-9 expression as well as clinical parameters with overall recurrence, univariate and multivariable Cox regression analyses were applied. In the univariate analysis, statistically significant associations with overall recurrence were observed for two parameters: MMP-2 expression (HR =2.99; 95% CI: 1.45–6.16, P=0.003) and MMP-9 expression (HR =2.40; 95% CI: 1.27–4.52, P=0.007) (Table 5). In the multivariate analyses, age (HR =0.53; 95% CI: 0.30–0.96, P=0.035), MMP-2 expression (HR =2.83; 95% CI: 1.37–5.85, P=0.005), and MMP-9 expression (HR =2.25; 95% CI: 1.19–4.24, P=0.013) showed statistically significant associations with overall recurrence (Table 5).
Table 5. Univariate and multivariable analysis for overall recurrence in pneumothorax patients.
| Variables | Item | Univariate | Multivariablea | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| Age (years) | >20 | 0.57 | (0.32, 1.01) | 0.056 | 0.53 | (0.30, 0.96) | 0.035 | |
| ≤20 | 1.00 | – | – | 1.00 | – | – | ||
| Sex | Female | 1.31 | (0.58, 2.94) | 0.514 | – | – | – | |
| Male | 1.00 | – | – | – | – | – | ||
| BMI (kg/m2) | ≥18 | 1.00 | (0.56, 1.79) | 0.999 | – | – | – | |
| <18 | 1.00 | – | – | – | – | – | ||
| Smoking status | Yes | 0.49 | (0.21, 1.17) | 0.107 | – | – | – | |
| No | 1.00 | – | – | – | – | – | ||
| Side involved | Unilateral | 1.25 | (0.66, 2.36) | 0.497 | – | – | – | |
| Bilateral | 1.00 | – | – | – | – | – | ||
| MMP-2 | High | 2.99 | (1.45, 6.16) | 0.003 | 2.83 | (1.37, 5.85) | 0.005 | |
| Low | 1.00 | – | – | 1.00 | – | – | ||
| MMP-9 | High | 2.40 | (1.27, 4.52) | 0.007 | 2.25 | (1.19, 4.24) | 0.013 | |
| Low | 1.00 | – | – | 1.00 | – | – | ||
a, variables with P values less than 0.1 in the univariate analysis were included in multivariable analysis. HR, hazard ratio; CI, confidence interval; BMI, body mass index; MMP-2, matrix metalloproteinase-2; MMP-9, matrix metalloproteinase-9.
Discussion
PSP is a troublesome and unpredictable disease and its recurrence is associated with the frequency of episodes (19). Surgery (VATS or open operation) remains an effective way to lower the recurrence rate (20-22). Several reports have recommended early operation for prevention of recurrence (23,24). Pulmonary high-resolution CT (HRCT) dystrophy severity score (DSS) scan findings can predict pneumothorax recurrence, and the risk of recurrence is related to the presence of blebs or bullae detected on HRCT (25). Recently, Primavesi et al. demonstrated that greater CT-based DSS values correlated with higher recurrence rates in conservatively treated patients (26). However, Ouanes-Besbes et al. (27) did not observe a significant correlation between CT-based DSS and recurrence. Therefore, the value of CT-based scoring systems in predicting PSP recurrence is still debatable. Until now, there are no clinical findings with enough sensitivity and specificity to predict PSP recurrence.
To the best of our knowledge, the present study is the only study to assess the influence of MMP-2 and MMP-9 on overall recurrence of PSP. While a previous study showed that MMP-2, MMP-9, MMP-7, and TIMP-2 were upregulated in PSP lesions without significant correlations with clinicopathological characteristics (28), our study demonstrated that the significantly increased MMP-2 and MMP-9 expression levels in pneumothorax tissues (Figure 1, Table 5) were independent factors of overall recurrence in PSP. Of note, the recurrence rates were high in our study, with 53.3% of patients (n=49) having recurrent PSP after first episode of PSP. The reason for the high recurrence rates was due to the fact that most of the patients presenting with a recurrence and primarily treated at nearby local hospitals were referred to our medical center for surgical treatment. Notably, our postoperative recurrence rate of pneumothorax is about 4.3%, lower than usual references (5–10%) (29). Therefore, the high ipsilateral pneumothorax recurrence rate (42.4%) was possibly due to the different therapeutic procedures being used including non-surgical management (bed rest, needle aspiration, tube thoracostomy) and surgical management.
Several factors, such as patient’s behavior (smoking) or status (age or BMI), have been found to be involved in pneumothorax recurrence propensity (2,30). Cigarette smoking is considered as a risk factor of PSP (30,31) and is an independent risk factor for recurrence (32). Alveolar macrophages from smokers express more MMP-9 than those from normal subjects (6). Smoking is also well known to be the major cause of COPD. Not only MMP-9, but also MMP-2, MMP-8, and MMP-12 are associated with COPD and emphysema in smokers (10,33,34). Inappropriate secretion of MMP-8, MMP-9, and MMP-12 by the stimulated inflammatory cells also play a role in asthmatic patients with smoking (35,36). Taken together, cigarette smoking could cause the accumulation and activation of the inflammatory cells and increase the expression of MMPs in various lung diseases. Our data showed that significantly increased expression levels of MMP-2 and MMP-9 in alveolar macrophages were observed in smokers (Tables 3,4), especially among male patients with PSP. A previous report has indicated that lower BMI is one of the risk factors for PSP (2). Lower BMI, defined as the body with greater height and lower weight, is prevalent in our PSP patients, both primary and recurrent (Tables 1,2).
In this study, we also noticed that most of the PSP patients were male (Table 1). Analysis in male (Table 4) but not in female patients (data not shown) revealed that recurrence rate and smoking status were significantly different between low and high MMP-2 or MMP-9 expression groups. These results indicated that male sex is still a risk factor for pneumothorax and subsequent recurrence, especially among male patients with high MMP-2 and MMP-9 expression in lung tissues.
In the multivariate Cox regression analysis (adjusted for age), the HR of recurrence in the age >20 group was 0.53 and that of recurrence in the age ≤20 group was 1.0 (Table 5), indicating that recurrence predominantly affects young subjects. Although PSP is not a life-threatening problem, it frequently recurs in young patients who are experiencing important life events and are expected to have normal physical activity. Therefore, evaluation of the risk factors for recurrence, including the analysis of MMP-2 and MMP-9 expression in young PSP patients, may provide useful information for physicians in clinical practice. Further large-scale clinical studies are required to reinforce the findings in our study.
In summary, using a cohort of 92 PSP patients, we observed a positive association between lung tissue MMP-2 and MMP-9 expression and the recurrence behavior. High MMP-2 and MMP-9 expression was found to be independently associated with overall recurrence, suggesting that inhibition of MMPs may be a promising way for reducing the risk of recurrence.
Acknowledgements
Funding: This work was supported by grants from the Kaohsiung Medical University Hospital (KMUH102-2M19, KMUH102-2M21, KMUH103-3M19, KMUH104-4M22) and Kaohsiung Municipal Ta-Tung Hospital (kmtth-104-013) of Taiwan.
Ethical Statement: This study was approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUH-IRB-20130268).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. 10.1136/thx.2010.136986 [DOI] [PubMed] [Google Scholar]
- 2.Guo Y, Xie C, Rodriguez RM, et al. Factors related to recurrence of spontaneous pneumothorax. Respirology 2005;10:378-84. 10.1111/j.1440-1843.2005.00715.x [DOI] [PubMed] [Google Scholar]
- 3.Sahn SA, Heffner JE. Spontaneous pneumothorax. N Engl J Med 2000;342:868-74. 10.1056/NEJM200003233421207 [DOI] [PubMed] [Google Scholar]
- 4.Schramel FM, Postmus PE, Vanderschueren RG. Current aspects of spontaneous pneumothorax. Eur Respir J 1997;10:1372-9. 10.1183/09031936.97.10061372 [DOI] [PubMed] [Google Scholar]
- 5.Goven D, Boutten A, Leçon-Malas V, et al. Induction of heme oxygenase-1, biliverdin reductase and H-ferritin in lung macrophage in smokers with primary spontaneous pneumothorax: role of HIF-1alpha. PLoS One 2010;5:e10886. 10.1371/journal.pone.0010886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lim S, Roche N, Oliver BG, et al. Balance of matrix metalloprotease-9 and tissue inhibitor of metalloprotease-1 from alveolar macrophages in cigarette smokers. Regulation by interleukin-10. Am J Respir Crit Care Med 2000;162:1355-60. 10.1164/ajrccm.162.4.9910097 [DOI] [PubMed] [Google Scholar]
- 7.De Smedt A, Vanderlinden E, Demanet C, et al. Characterisation of pleural inflammation occurring after primary spontaneous pneumothorax. Eur Respir J 2004;23:896-900. 10.1183/09031936.04.00079304 [DOI] [PubMed] [Google Scholar]
- 8.Tetley TD. New perspectives on basic mechanisms in lung disease. 6. Proteinase imbalance: its role in lung disease. Thorax 1993;48:560-5. 10.1136/thx.48.5.560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vu TH, Werb Z. Matrix metalloproteinases: effectors of development and normal physiology. Genes Dev 2000;14:2123-33. 10.1101/gad.815400 [DOI] [PubMed] [Google Scholar]
- 10.Chakrabarti S, Patel KD. Matrix metalloproteinase-2 (MMP-2) and MMP-9 in pulmonary pathology. Exp Lung Res 2005;31:599-621. 10.1080/019021490944232 [DOI] [PubMed] [Google Scholar]
- 11.Ohbayashi H. Matrix metalloproteinases in lung diseases. Curr Protein Pept Sci 2002;3:409-21. 10.2174/1389203023380549 [DOI] [PubMed] [Google Scholar]
- 12.Cathcart J, Pulkoski-Gross A, Cao J. Targeting Matrix Metalloproteinases in Cancer: Bringing New Life to Old Ideas. Genes Dis 2015;2:26-34. 10.1016/j.gendis.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chetty A, Cao GJ, Severgnini M, et al. Role of matrix metalloprotease-9 in hyperoxic injury in developing lung. Am J Physiol Lung Cell Mol Physiol 2008;295:L584-92. 10.1152/ajplung.00441.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sweet DG, Curley AE, Chesshyre E, et al. The role of matrix metalloproteinases -9 and -2 in development of neonatal chronic lung disease. Acta Paediatr 2004;93:791-6. 10.1111/j.1651-2227.2004.tb03020.x [DOI] [PubMed] [Google Scholar]
- 15.Montaño M, Beccerril C, Ruiz V, et al. Matrix metalloproteinases activity in COPD associated with wood smoke. Chest 2004;125:466-72. 10.1378/chest.125.2.466 [DOI] [PubMed] [Google Scholar]
- 16.Chou SH, Li HP, Lee JY, et al. Needlescopic video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2009;18:221-4. 10.1080/13645700802649425 [DOI] [PubMed] [Google Scholar]
- 17.Chou SH, Li HP, Lee JY, et al. Is prophylactic treatment of contralateral blebs in patients with primary spontaneous pneumothorax indicated? J Thorac Cardiovasc Surg 2010;139:1241-5. 10.1016/j.jtcvs.2009.07.047 [DOI] [PubMed] [Google Scholar]
- 18.Chou SH, Chuang IC, Huang MF, et al. Comparison of needlescopic and conventional video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim Invasive Ther Allied Technol 2012;21:168-72. 10.3109/13645706.2011.580763 [DOI] [PubMed] [Google Scholar]
- 19.Ng CS, Lee TW, Wan S, et al. Video assisted thoracic surgery in the management of spontaneous pneumothorax: the current status. Postgrad Med J 2006;82:179-85. 10.1136/pgmj.2005.038398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. 10.1016/S0140-6736(07)61163-5 [DOI] [PubMed] [Google Scholar]
- 21.Lang-Lazdunski L, de Kerangal X, Pons F, et al. Primary spontaneous pneumothorax: one-stage treatment by bilateral videothoracoscopy. Ann Thorac Surg 2000;70:412-7. 10.1016/S0003-4975(00)01552-6 [DOI] [PubMed] [Google Scholar]
- 22.Lang-Lazdunski L, Chapuis O, Bonnet PM, et al. Videothoracoscopic bleb excision and pleural abrasion for the treatment of primary spontaneous pneumothorax: long-term results. Ann Thorac Surg 2003;75:960-5. 10.1016/S0003-4975(02)04544-7 [DOI] [PubMed] [Google Scholar]
- 23.Ben-Nun A, Soudack M, Best LA. Video-assisted thoracoscopic surgery for recurrent spontaneous pneumothorax: the long-term benefit. World J Surg 2006;30:285-90. 10.1007/s00268-005-0235-2 [DOI] [PubMed] [Google Scholar]
- 24.Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopic surgery for primary spontaneous pneumothorax: evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005;127:2226-30. 10.1378/chest.127.6.2226 [DOI] [PubMed] [Google Scholar]
- 25.Casali C, Stefani A, Ligabue G, et al. Role of blebs and bullae detected by high-resolution computed tomography and recurrent spontaneous pneumothorax. Ann Thorac Surg 2013;95:249-55. 10.1016/j.athoracsur.2012.05.073 [DOI] [PubMed] [Google Scholar]
- 26.Primavesi F, Jäger T, Meissnitzer T, et al. First Episode of Spontaneous Pneumothorax: CT-based Scoring to Select Patients for Early Surgery. World J Surg 2016;40:1112-20. 10.1007/s00268-015-3371-3 [DOI] [PubMed] [Google Scholar]
- 27.Ouanes-Besbes L, Golli M, Knani J, et al. Prediction of recurrent spontaneous pneumothorax: CT scan findings versus management features. Respir Med 2007;101:230-6. 10.1016/j.rmed.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 28.Chen CK, Chen PR, Huang HC, et al. Overexpression of matrix metalloproteinases in lung tissue of patients with primary spontaneous pneumothorax. Respiration 2014;88:418-25. 10.1159/000366065 [DOI] [PubMed] [Google Scholar]
- 29.Luh SP. Review: Diagnosis and treatment of primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2010;11:735-44. 10.1631/jzus.B1000131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bense L, Eklund G, Wiman LG. Smoking and the increased risk of contracting spontaneous pneumothorax. Chest 1987;92:1009-12. 10.1378/chest.92.6.1009 [DOI] [PubMed] [Google Scholar]
- 31.Cheng YL, Huang TW, Lin CK, et al. The impact of smoking in primary spontaneous pneumothorax. J Thorac Cardiovasc Surg 2009;138:192-5. 10.1016/j.jtcvs.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 32.Lippert HL, Lund O, Blegvad S, et al. Independent risk factors for cumulative recurrence rate after first spontaneous pneumothorax. Eur Respir J 1991;4:324-31. [PubMed] [Google Scholar]
- 33.Ilumets H, Rytilä P, Demedts I, et al. Matrix metalloproteinases -8, -9 and -12 in smokers and patients with stage 0 COPD. Int J Chron Obstruct Pulmon Dis 2007;2:369-79. [PMC free article] [PubMed] [Google Scholar]
- 34.Wu Y, Li J, Wu J, et al. Discovery of potent and selective matrix metalloprotease 12 inhibitors for the potential treatment of chronic obstructive pulmonary disease (COPD). Bioorg Med Chem Lett 2012;22:138-43. 10.1016/j.bmcl.2011.11.046 [DOI] [PubMed] [Google Scholar]
- 35.Gueders MM, Foidart JM, Noel A, et al. Matrix metalloproteinases (MMPs) and tissue inhibitors of MMPs in the respiratory tract: potential implications in asthma and other lung diseases. Eur J Pharmacol 2006;533:133-44. 10.1016/j.ejphar.2005.12.082 [DOI] [PubMed] [Google Scholar]
- 36.Chaudhuri R, McSharry C, Brady J, et al. Sputum matrix metalloproteinase-12 in patients with chronic obstructive pulmonary disease and asthma: relationship to disease severity. J Allergy Clin Immunol 2012;129:655-63.e8. 10.1016/j.jaci.2011.12.996 [DOI] [PubMed] [Google Scholar]