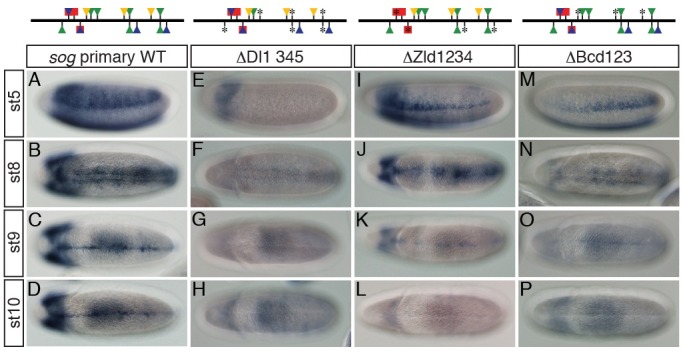
Fig. 3. Intact activity of the sog primary enhancer in the presumptive neurogenic ectoderm is required for its late activity in the ventral midline of the developing Drosophila embryo. Approximately 2-10 h AED embryos were collected, dechorionated, fixed, and hybridized with DIG-UTP labeled antisense lacZ RNA. Binding sites of Dl (green), Zld (blue), Sna (red), and Bcd (yellow) in the sog primary enhancer are depicted on top of each column. Triangles and squares represent binding sites for a transcriptional activator and repressor, respectively. Mutagenized Dl-, Zld-, Sna-, and Bcd-binding sites are marked with asterisks (*). (A-D) The wild-type (WT) sog primary enhancer directs lacZ expression in the early neurogenic ectoderm and the late ventral midline of Drosophila embryo. (E-H) Mutations in four Dl-binding sites abolished lacZ expression both early in the neurogenic ectoderm and late in the ventral midline. (I-L) Lack of Zld-binding sites in the primary enhancer led not only to severe reduction in lacZ expression width in the neurogenic ectoderm, but also to complete loss of lacZ expression in the ventral midline. Note that the remaining lacZ expression also gradually diminished along the anterior-posterior (AP) axis (I). (M-P) Removal of Bcd-binding sites in the primary enhancer produced similar lacZ expression patterns to those of the primary enhancer containing no Zld-binding site, except that narrow lacZ expression gradually increased from the anterior to posterior pole. This lacZ pattern appears to be a mirror image of that produced by the △Zld1234 construct (compare M with I).