Abstract
Background
Early antiretroviral therapy (ART) initiation in HIV-infected infants significantly improves survival but is often delayed in resource-limited settings. Adding HIV testing of infants at birth to the current recommendation of testing at age 4–6 weeks may improve testing rates and decrease time to ART initiation. We modeled the benefit of adding HIV testing at birth to the current 6-week testing algorithm.
Methods
Microsoft Excel was used to create a decision-tree model of the care continuum for the estimated 1,400,000 HIV-infected women and their infants in sub-Saharan Africa in 2012. The model assumed average published rates for facility births (42.9%), prevention of mother-to-child HIV transmission utilization (63%), mother-to-child-transmission rates based on prevention of mother-to-child HIV transmission regimen (5%–40%), return of test results (41%), enrollment in HIV care (52%), and ART initiation (54%). We conducted sensitivity analyses to model the impact of key variables and applied the model to specific country examples.
Results
Adding HIV testing at birth would increase the number of infants on ART by 204% by age 18 months. The greatest increase is seen in early ART initiations (543% by age 3 months). The increase would lead to a corresponding increase in survival at 12 months of age, with 5108 fewer infant deaths (44,550, versus 49,658).
Conclusion
Adding HIV testing at birth has the potential to improve the number and timing of ART initiation of HIV-infected infants, leading to a decrease in infant mortality. Using this model, countries should investigate a combination of HIV testing at birth and during the early infant period.
Keywords: HIV testing, modeling, early infant diagnosis
BACKGROUND
In 2012, an estimated 260,000 children were newly infected with HIV worldwide1; approximately 90% of these infections occurred in sub-Saharan Africa through mother-to-child transmission (MTCT).2 Without antiretroviral therapy (ART), 52% of HIV-infected infants die within 12 months after birth,3 with peak mortality occurring at 3 months of age.4 Fortunately, survival rates improve greatly with ART, and studies have shown that infants initiated on ART before 12 weeks of age have up to a 76% reduction in mortality compared with infants in whom ART was delayed until a threshold CD4 percentage or presentation with a World Health Organization (WHO) clinical stage.5-7
Current WHO guidelines for resource-limited settings recommend virologic testing for HIV-exposed infants at 4–6 weeks of age, but also allow for consideration of the addition of testing at birth.8 In resource-rich settings such as the United States, however, the standard practice is to provide virologic testing at multiple time points with the first as early as 14 days or at birth for infants considered to be at high risk of HIV infection.9 There are 3 main rationales for WHO’s recommendation of 6-week testing in resource-limited settings: first, this timing coincides with the first scheduled infant vaccination visit at age 6 weeks; second, HIV DNA polymerase chain reaction (PCR) testing at 6 weeks is greater than 95% sensitive for all perinatal HIV transmissions (defined as in-utero or intrapartum transmission), which is higher than the sensitivity of HIV DNA PCR testing at birth10-12; and third, testing at age 6 weeks can also detect a majority of early postnatal HIV transmissions (defined as transmission through breastfeeding).13 However, in practice, early infant diagnosis (EID) services are often not available, are poorly functioning, and have low follow-up rates; in 2012, only 39% of HIV-exposed infants in resource-limited settings had an HIV DNA PCR test performed within 2 months of birth.1 Furthermore, even for those infants who receive testing, there are delays in treatment initiation once a positive result is returned to the health care provider. Recent studies have shown that average age of ART initiation among HIV-infected infants diagnosed through EID services ranges widely, from 2 months to as long as 11 months.14-18 Retaining HIV-exposed infants in care, returning HIV test results to their caregivers, and providing early ART to HIV-infected infants continue to be major challenges for prevention of mother-to-child HIV transmission (PMTCT) and pediatric HIV programs.19
One proposal for increasing early HIV testing and decreasing the time to infant ART initiation in resource-limited settings involves conducting the first HIV DNA PCR test shortly after birth.8,20 In this scenario, dried blood spot samples would be collected from all HIV-exposed infants born in health care facilities before discharge. HIV DNA PCR test results would be returned at the first scheduled postnatal vaccination visit at age 6 weeks. This approach offers several potential benefits. First, the number and proportion of HIV-exposed infants in resource-limited settings who actually receive HIV testing could increase if HIV testing was included in the routine neonatal package of care currently offered after delivery. Second, for those who test positive, referral to HIV care services could be initiated at the time of the first immunization visit, which would potentially allow for treatment to be initiated at least 4 to 6 weeks earlier than with the current testing algorithm, even without improvements in specimen transport and laboratory processing turnaround time. Finally, infants infected in-utero are known to have the most rapid progression of disease and would be the ones specifically identified through birth testing, permitting early life saving treatment for this highly vulnerable population.21 Despite these potential benefits, there are currently limited data on the impact of a newborn HIV testing program in resource-limited settings. To provide insight into this question, we modeled the use of HIV DNA PCR testing at birth to assess its impact on the number of HIV-infected infants initiated on treatment by ages 3 and 18 months and the potential impact on HIV-related mortality.
METHODS
We used Microsoft Excel to create a decision-tree model of the continuum of care for HIV-infected women and their infants in sub-Saharan Africa (Table 1). Our model included decision points for HIV-infected pregnant women during receipt of antenatal services through delivery and for their infants from birth through breastfeeding until 18 months of age. We searched PubMed and abstracts from relevant meetings [Conference on Retroviruses and Opportunistic Infections (CROI) and the International AIDS Society (IAS)] to identify rates from sub-Saharan Africa for each of the decision branch points along the cascade; we used a conservative median estimate for the model and conducted sensitivity analyses on assumptions used to gauge the effect of changing assumptions.
TABLE 1.
Assumption Values, Including High and Low Estimates of Each Model Parameter Used in Sensitivity Analysis, and Source
| Parameter | Average Value, % | Range | Source |
|---|---|---|---|
| Facility births | 42.9 | 14%–90% | Montagu et al22 |
| PMTCT utilization | UNAIDS Global Report 2013 | ||
| Highly effective regimen | 63 | 7%–95% | |
| None | 37 | ||
| HIV transmission rates | Working Paper on Mother-to- Child HIV Transmission Rates for use in Spectrum 2011 | ||
| Highly effective regimen | 5.6 | ||
| Slightly effective regimen | 30.3 | ||
| None | 40.3 | ||
| Timing of transmission without PMTCT* | Cavarelli et al23 | ||
| Peripartum (in-utero and intrapartum) | 70 | ||
| Postpartum | 30 | ||
| Timing of transmission with PMTCT | Kesho Bora Study Group 2011 | ||
| Peripartum (in-utero and intrapartum) | 57 | ||
| Postpartum | 43 | ||
| At birth survival | Chen et al24 | ||
| Baseline for HIV+ infants | 96 | ||
| On PMTCT therapy | 94 | ||
| HIV testing rate | WHO Vaccine Preventable Disease Monitoring System 2012; UNAIDS Global Report 2013 | ||
| Newborn testing | 80 | 78%–86% | |
| 6-wk testing | 39 | 3%–95% | |
| HIV DNA PCR test sensitivity | Lilian et al12; Neilsen-Saines et al25; Burgard et al26; and Shapiro et al27 | ||
| Newborn testing | 60 | 50%–70% | |
| 6-wk testing | 100 | ||
| Return of test eesults | WHO Vaccine Preventable Disease Monitoring System 2012; estimated range of ±15%; Ciaranello et al19; Anoje et al28; Coulibaly et al16; Hsiao et al29; Hassan et al30; and Motswere- Chirwa et al31 | ||
| Newborn testing | 71 | 56%–86% | |
| 6-wk testing | 52 | 45%–99% | |
| Enrollment into care | 52 | 30%–78% | Ciaranello et al19; Coulibaly et al16; and Motswere-Chirwa et al31 |
| Initiation of ART | 54 | 38%–77% | Ciaranello et al19; Braun et al14; Motswere-Chirwa et al31; and Innes et al17 |
| Treatment within 3 mo of life | Braun et al14;Motswere-Chirwa et al31; and Innes et al17 | ||
| Newborn testing | 37.5 | ||
| 6-wk testing | 8 | ||
| Treatment within 6 mo of life | Braun et al14; Motswere-Chirwa31; and Innes et al17 | ||
| Newborn testing | 59 | ||
| 6-wk testing | 54 | ||
| Treatment within 12 mo of life | Braun et al14; Motswere-Chirwa et al31; and Innes et al17 | ||
| Newborn testing | 80 | ||
| 6-wk testing | 75 | ||
| Postpartum transmission timing | Nduati et al32 | ||
| within 6 wk | 63 | ||
| within 9 mo | 81 | ||
| within 18 mo | 100 | ||
| 1 yr mortality | Marston et al51; and Violari et al5 | ||
| Peripartum no treatment | 48 | ||
| Postpartum no treatment | 24 | ||
| On delayed treatment | 16 | ||
| On treatment by 3 mo | 4 |
PMTCT defined as WHO Option A or B.
Assumptions for Pregnancy, Delivery, and Early MTCT
Our model was constructed using a theoretical cohort of 1,400,000 HIV-infected pregnant women, which is the number of HIV-infected pregnant women estimated to be living in sub-Saharan Africa in 2012.1 Using published data for sub-Saharan Africa, we assumed that 43% of births occurred in a health facility22; this was assumed to be the same for HIV-infected and HIV-uninfected women. We modeled HIV transmission using varying rates of transmission depending on type of PMTCT regimen received. We estimated that 63% of all HIV-positive pregnant women would receive a highly effective PMTCT regimen (WHO Option A or B) and that the remaining women received no PMTCT interventions.1 The latest estimates of PMTCT utilization do not report single-dose nevirapine (sd-NVP) usage; however, as this regimen is still used in some settings in sub-Saharan Africa, we included it in the sensitivity analysis section of our model and used the most recently published estimate of sd-NVP use.33 Transmission rates for highly effective PMTCT, sd-NVP, and no PMTCT were estimated at 5.6%, 30.3%, and 40.3%, respectively, and incorporated the risk of postpartum transmission during 18 months of breastfeeding.33-35 We assumed that PMTCT intervention would affect the proportion of infant infections occurring perinatally; with 57% of HIV infections occurring perinatally as opposed to postpartum when a PMTCT intervention was provided compared with 70% when a woman received no PMTCT intervention.23,36 In total, this meant that 67.5% of HIV-positive infants in our model were infected perinatally.
Assumptions for HIV Testing of Exposed Infants at Birth
For our model of newborn HIV testing, we estimated that 80% of HIV-exposed infants born in facilities would have a dried blood spot sample for HIV DNA PCR test collected before discharge. This estimate was based on uptake of BCG vaccination, another service provided at birth in resource-limited settings.37 Although uptake rates for HIV-related interventions may differ from uptake of other health interventions, multiple studies of HIV testing rates among pregnant women during delivery have shown that more than 85% of women accept testing for themselves38,39; acceptance rates for infant HIV testing offered to mothers in outpatient clinics are even higher.40
We assumed that HIV DNA PCR testing at birth would detect 60% of all infections acquired perinatally, but no postnatal infections. This sensitivity was based on data demonstrating that 68% of perinatally infected infants identified by HIV DNA PCR testing at 6 weeks of age had also tested positive at birth, and other studies that tested infants at birth that showed lower sensitivities.12,25-27,41 Because we assumed that newborn test results would be returned at the first regularly scheduled vaccination visit at 6 weeks, we used vaccine uptake as a proxy of receipt of results. However, in our model, we used the 77% average rate of completion of the diphtheria-tetanus-pertussis (DTP) series at age 14 weeks rather than the 85% rate of uptake of the first DTP vaccination to try to account for potential loss-to-follow-up of infants whose mothers chose to receive vaccination services at sites different from delivery sites.37 One hindrance to receipt of results is long turnaround time of PCR testing, as samples are usually sent offsite to be performed. A review of studies showed that turnaround time can range from as little as 7 days to as long as 92 days.19,28,42-46 The weighted average of time was 28 days, and if using that assumption, all tests would be returned in time for the 6-week visit. However, pooling all studies, 20% of infants would not receive results by 6 weeks, and the model assumed that only 80% of results would be available at 6 weeks.
Assumptions for HIV Testing at 6 Weeks
We assumed that 39% of all HIV-exposed infants received HIV DNA PCR testing at 6 weeks and that this test had a sensitivity of 100% for perinatal HIV infections.1,47 We estimated that 63% of all postnatal transmissions would also be detected at age 6 weeks, based on data showing that HIV transmission is higher in the first 6 months of breastfeeding and gradually decreases over time.32
Assumptions for HIV Testing After 6 Weeks
We assumed that HIV-exposed breastfeeding infants who initially tested negative, regardless of the timing of the first test, had an additional HIV DNA PCR test schedule at 9 months, consistent with WHO recommendations.8 Our model assumed that 16% would not return for the 9-month test, consistent with the difference between 6-week DTP vaccination and 9-month measles vaccination rates. We also assumed that 81% of postnatal infections in a breastfeeding infant would have occurred by that age and that all infections could be detected by a DNA PCR test.32 For the infants infected after 9 months of age, the final diagnostic test would be performed at 18 months, which was assumed to be the end of the breastfeeding period. Rates were assumed to be quite low, consistent with the 11% repeat measles vaccination, also given at 18 months. All infections of infants who would receive this test were considered to be detectable by rapid HIV antibody testing at this time point.
Assumptions for Enrollment in HIV Care and Treatment
Based on current published data on linkage to care for HIV-infected infants in sub-Saharan Africa, we estimated that 52% of infants identified as HIV infected would enroll into care,16,19,28-31 and 54% of those enrolled into care would initiate ART.16,19,31 To determine the age at ART initiation, we assumed a median time of 10 weeks from the time of HIV diagnosis to the time of ART initiation.14,17,31 We used these same assumptions regardless of the testing algorithm or the infant’s age at HIV diagnosis.
Estimates of Infant Survival
To estimate the survival of HIV-infected infants, we assumed that HIV-infected women experienced rates of stillbirths of 2% for those not receiving any PMTCT and 4% for those receiving PMTCT.24 Among HIV-infected infants not receiving ART, we estimated a 12-month mortality rate of 52% for perinatally infected infants and 26% for postnatally infected infants.3 Among HIV-infected infants receiving ART, we estimated 12-month mortality rates of 4% for those initiating ART before age 3 months and 16% for those initiating ART after age 3 months.3,5
Model Endpoints
Our model endpoints were the number of infants initiated on ART by ages 3 and 18 months and the number of HIV-related deaths prevented by 12 months. We compared these endpoints for infants who had a 6-week HIV DNA PCR test and for infants who had additional newborn DNA PCR testing.
Sensitivity Analysis and Country Examples
A 1-way sensitivity analysis was conducted for key variables in the model to determine their influence on the model endpoints. For this sensitivity analysis, we used the higher and lower published estimates of the following variables: facility births, PMTCT coverage, sd-NVP usage, newborn HIV testing rates, newborn HIV DNA PCR sensitivity, receipt of newborn test results, 6-week HIV testing rates, receipt of 6-week HIV test results, enrollment into care, and initiation of treatment (Table 2). We calculated outcomes using country-specific data for these key variables in 2 countries, Kenya and Swaziland, to illustrate the potential impact of newborn HIV testing in 2 sub-Saharan African settings with different HIV epidemics and health care systems (Table 3).
TABLE 2.
Sensitivity Analysis of the Impact of the Highest and Lowest Published Value for Model Parameters on the Number of HIV-Infected Infants Receiving ART by Age 3 Months, HIV-Infected Infants Receiving ART by Age 18 Months, and HIV-Related Deaths Prevented
| Model Parameters | Low Value* | High Value* | Infants Receiving ART by Age 3 mo
|
Infants Receiving ART by Age 18 mo
|
Deaths Prevented
|
|||
|---|---|---|---|---|---|---|---|---|
| With Low Value | With High Value | With Low Value | With High Value | With Low Value | With High Value | |||
| % of births occurring at health care facilities | 14 | 90 | −67 | 110 | −67 | 110 | −67 | 110 |
| % receiving sd-NVP | 0 | 11 | 0 | −8 | 0 | −33 | 0 | −6 |
| % receiving highly effective PMTCT | 7 | 84 | 109 | −41 | 107 | −40 | 107 | −40 |
| % of HIV-exposed infants receiving HIV DNA PCR testing at birth | 78 | 86 | −2 | 6 | −2 | 5 | −3 | 10 |
| Newborn test sensitivity†, % | 50 | 70 | −14 | 14 | −10 | 10 | −19 | 14 |
| % of infants whose mothers receive newborn HIV DNA PCR test results | 56 | 86 | −16 | 16 | −13 | 13 | −25 | 25 |
| % of HIV-exposed infants receiving HIV DNA PCR testing at age 6 wk | 3 | 95 | −8 | 13 | −22 | 40 | 39 | −62 |
| % of infants whose mothers receive 6-wk HIV DNA PCR test results | 45 | 99 | −1 | 8 | −3 | 22 | 6 | −39 |
| % of HIV-infected infants enrolled into HIV care | 30 | 78 | −42 | 50 | −42 | 50 | −42 | 50 |
| % of enrolled HIV-infected infants started on treatment | 38 | 77 | −29 | 43 | −29 | 43 | −29 | 43 |
Lowest and highest values found in published studies or country reports.
Newborn test sensitivity is defined as percent of infants testing positive for HIV at six weeks who also tested positive at birth.
TABLE 3.
Key Variables for Models of the Impact of Newborn HIV Testing in Kenya and Swaziland
| Variable | Kenya | Swaziland |
|---|---|---|
| Number of HIV-infected pregnant women1 | 86,000 | 12,000 |
| % of births occurring at health care facilities22 | 41 | 74 |
| Uptake of highly effective PMTCT* regimen among pregnant women,1 % | 53 | 83 |
| % of HIV-exposed infants receiving HIV DNA PCR testing at 2 mo1 | 39 | 81 |
| % of infants whose mothers receive 6-wk HIV DNA PCR test results48 | 71 | 50 |
| % of HIV-infected infants enrolled into HIV care48 | 42 | 75 |
| % of HIV-infected infants who initiate ART1,48 | 49 | 59 |
PMTCT.
RESULTS
In our model, 600,600 (43%) of 1,400,000 HIV-infected women delivered in a health facility. Of these women, 378,378 (63%) received a WHO highly effective PMTCT regimen and 222,222 (37%) did not receive any PMTCT intervention. Among live births, 71,514 (67%) infants were infected with HIV perinatally and an additional 34,377 (33%) were infected postnatally.
Compared with the standard 6-week HIV testing algorithm, the addition of DNA PCR testing at birth increased the number of infants initiated on ART at age 3 months by 543% (1907 with newborn and 6-week testing versus 351 with 6-week testing alone) and increased the number of infected infants initiated on ART by age 18 months by 209% (9141 versus 4372, respectively) (Fig. 1). The increase in the number of infants receiving early ART led to a corresponding increase in survival at 12 months of age, with 5108 fewer infant deaths (49,658 versus 44,550) as a result of earlier HIV diagnosis and ART initiation.
FIGURE 1.
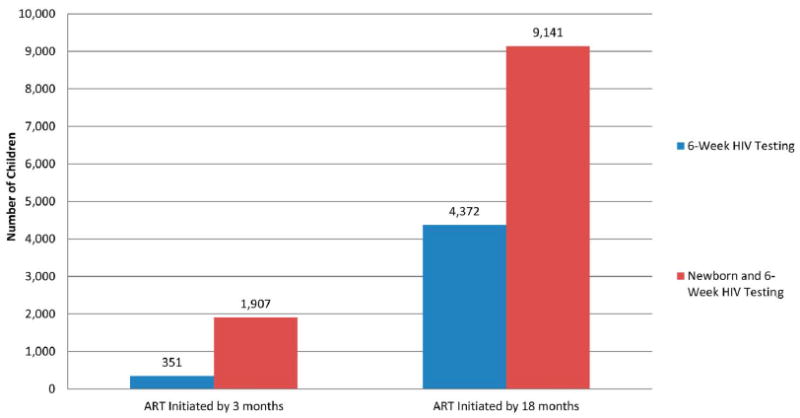
Model estimates of the number of children initiated on ART by 3 and 18 months with current testing practices and with the addition of newborn testing.
The sensitivity analysis showed that facility birth rates and enrollment in care are the 2 decision-tree points that have the most effect on the impact of newborn HIV testing (Figs. 2A–C). When facility birth rates reached 90%, as they are in the Republic of Congo,22 as opposed to the average of 43% across sub-Saharan Africa, the number of infants on ART by 3 months using newborn HIV testing increased by 110% (2093 additional infants) and the number of deaths prevented increased by 110% (1877 additional lives saved) (Figs. 2A–C). The next largest influencer on the impact of newborn HIV testing was utilization of PMTCT. Here, the relationship was inverse, as the lower the usage of PMTCT, and correspondingly a larger number of peripartum transmissions, the larger the impact of newborn testing; if rates were as low as 6%, as they are in Nigeria (1), then the number of infants on ART by 3 months increased by 109% (2071 additional infants), leading to a 107% increase in deaths prevented (1828 additional lives saved) (Figs. 2A–C).
FIGURE 2.
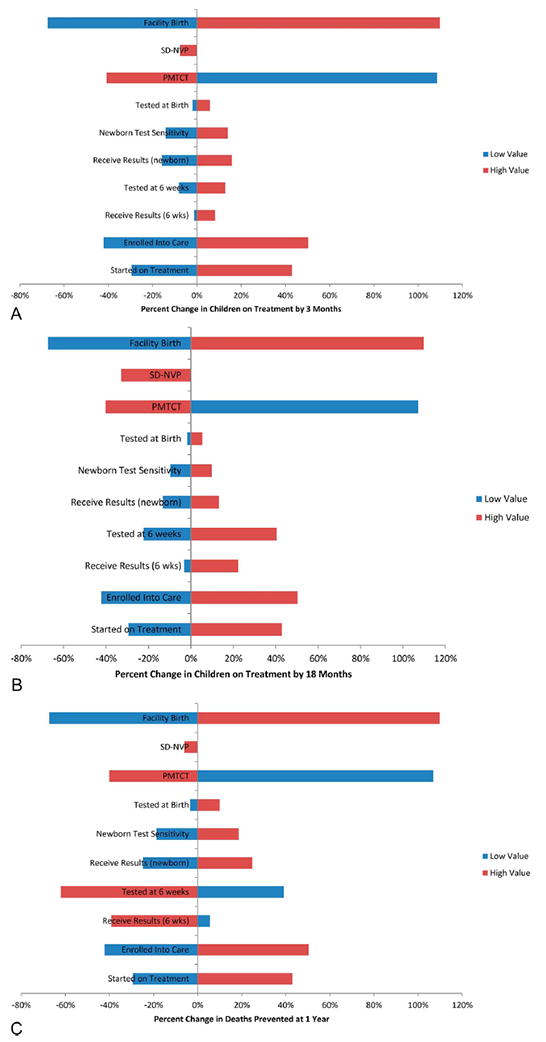
A, Sensitivity analysis of the impact of newborn testing in settings with high and low estimates of key variables in the model compared with average estimates in percent change of infants on ART by age 3 months. B, Sensitivity analysis of the impact of newborn testing in settings with high and low estimates of key variables in the model compared with average estimates in percent change of infants on ART by 18 months. C, Sensitivity analysis of the impact of newborn testing in settings with high and low estimates of key variables in the model compared with average estimates in percent change in number of additional infant deaths prevented at age 12 months.
Country Examples
Because of the wide variations in program performance across sub-Saharan Africa and the impact of various program factors on the outcome of the model, we sought to apply the model to specific country contexts. We used published data from Swaziland and Kenya to highlight the difference that key variables could have on the impact of newborn HIV testing. Compared with Kenya, Swaziland has a higher facility birth rate (74% versus 41%), higher HIV prevalence (31% versus 6.2%), and a higher current rate of 6-week HIV testing (81% versus 39%). Swaziland also has a much higher PMTCT utilization rate (83% versus 53%) (Table 3). Using these country-specific assumptions, our model showed that newborn HIV testing would have a larger impact in Kenya, increasing the number of infants on ART at age 3 months by 359% compared with 249% in Swaziland (Table 4). This translates into 66 more infant deaths prevented each year in Kenya using newborn testing compared with 9 infant deaths per year prevented using newborn testing in Swaziland (Table 4).
TABLE 4.
Infant ART Initiation at 3 and 18 Months, and Lives Saved by Adding Newborn Testing to Routine Infant HIV Testing Algorithms in Kenya and Swaziland
| Increase in Infants on Treatment by 3 mo | Increase in Infants on Treatment by 18 mo | Additional Lives Saved per Year | |
|---|---|---|---|
| Kenya | 78 (359%) | 178 (147%) | 66 |
| Swaziland | 18 (249%) | 22 (113%) | 9 |
DISCUSSION
Our model shows that newborn HIV DNA PCR testing in sub-Saharan Africa has the potential to increase the number of infected infants initiated on treatment before the age of 3 months and significantly reduce infant mortality due to perinatally acquired HIV; the addition of newborn HIV testing would increase the number of infants on ART by 3 months by 543% and help prevent 5108 HIV-related infant deaths at 12 months of age. Although this model provides insight into the potential impact of newborn testing in sub-Saharan Africa, the real impact of newborn testing will be highly variable based on a country’s specific burden of HIV and program performance. Our sensitivity analysis showed that newborn testing would have the largest impact and prevent the most deaths in countries with a high proportion of facility births and the countries with the lowest PMTCT rates. In our country examples, although Swaziland has stronger systems once infected children are identified, the fact that Kenya has lower PMTCT rates, translating into more peripartum infections, increases the potential of newborn testing to find more HIV-infected infants in that country.
Our model has several limitations. First, our model assumptions were based on published data on the cascade of care for HIV-infected women and their infants across sub-Saharan Africa. The published rates may not reflect the most recent performance and may present a biased picture of program performance by ignoring improvements in care delivery that have been made in recent years. Although we used the most recent data available at the time of our analysis, new data have likely become available in the interim that could change the modeled impact of birth testing. Second, our model assumptions about uptake of newborn HIV testing and return of test results were based on data from usage of other services, including vaccination services. One of the largest programmatic hurdles for a successful newborn HIV testing program would be the return of results at the 6-week immunization visit, especially as immunization services are often more decentralized than maternity facilities and that women may choose not to return to the same clinic where they delivered for their infant’s immunizations. A study to better estimate these rates and understand the programmatic feasibility, including costs, of newborn testing is needed. Third, many of our assumptions are derived from studies that did not focus on HIV-infected women who deliver in health care facilities. The women who choose to deliver in a health care facility may not be representative of the larger population of HIV-infected women, as they may have greater access to health care and may be more likely to use PMTCT and HIV-exposed infant follow-up services including EID. In our model, we assumed that HIV-infected and HIV-uninfected women would deliver in health facilities at the same rates; however, as HIV is often more common in urban areas, where the density of health facilities is higher and where women have higher uptake of facility births, HIV-infected women may be more likely to deliver in health care facilities, thus underestimating the effect of newborn testing. Our model could not account for these issues, mainly because this type of stratified data is not available. Finally, newborn testing would not replace the need for an additional HIV DNA PCR test at a later age, as false negatives could occur, a number of intrapartum infections would be missed, and early breastfeeding transmissions would not be detected. Therefore, if a country implemented a newborn HIV testing model, infants testing negative at birth would require at least 2 HIV DNA PCR tests, meaning that additional commodities and laboratory capacity would be required. A formal costing analysis of newborn HIV testing is needed to inform international and country-specific decisions regarding the feasibility of implementing a newborn HIV testing program.
Newborn testing should continue to be evaluated as a potential addition to other strategies for earlier HIV diagnosis and treatment initiation in infants. Pilot studies and operational research are urgently needed to better determine the true impact that this strategy could have, and how postnatal transmission changes might influence the timing of subsequent testing, especially in settings where PMTCT services, including universal ART for life for HIV-infected pregnant and breastfeeding women, are rapidly expanding.49 Small-scale trials of newborn HIV testing in delivery wards could help verify key model assumptions, such as acceptance of newborn HIV testing and receipt of HIV test results at the 6-week vaccination visit. These pilot projects would also help identify unforeseen logistical obstacles to newborn testing and assess the feasibility of coordination between HIV testing in the delivery ward and return of results at an immunization clinic.
In addition to pilot testing, additional modeling should be performed to account for the introduction of new technologies, such as ultrasensitive and point-of-care HIV DNA PCR tests or PMTCT program improvements. In addition, new algorithms for infant HIV testing should be explored, particularly combinations of newborn testing with a second HIV test that could be performed at a time later than the 6-week visit to capture more postnatal transmissions; 10 weeks has been suggested.50 Further modeling is needed to identify an optimal testing algorithm that maximizes both timely HIV diagnosis and infant lives saved and that minimizes cost. Finally, as we have shown, the impact of newborn testing is highly context dependent, meaning that countries and even individual health centers need to consider their individual program context as they evaluate the utility of newborn HIV testing.
Acknowledgments
This publication was made possible by support from the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) through the U.S. Centers for Disease Control and Prevention (CDC), Division of Global HIV/ AIDS (DGHA).
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
Footnotes
The authors have no funding or conflicts of interest to disclose.
References
- 1.UNICEF. Towards an AIDS-Free Generation—Children and AIDS Sixth Stocktaking Report 2013. New York: UNICEF; 2013. [Google Scholar]
- 2.Torpey K, Kasonde P, Kabaso M, et al. Reducing pediatric HIV infection: estimating mother-to-child transmission rates in a program setting in Zambia. J Acquir Immune Defic Surg. 2010;54:415–422. doi: 10.1097/QAI.0b013e3181e36616. [DOI] [PubMed] [Google Scholar]
- 3.Becquet R, Marston M, Dabis F, et al. Children who acquire HIV infection perinatally are at higher risk of early death than those acquiring infection through breastmilk: a meta-analysis. PLoS One. 2012;7:e28510. doi: 10.1371/journal.pone.0028510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newell M, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 5.Violari A, Cotton MF, Gibb DM, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luzuriaga K, McManus M, Mofenson L, et al. A trial of three antiretroviral regimens in HIV-1-infected children. N Engl J Med. 2004;350:2471–2480. doi: 10.1056/NEJMoa032706. [DOI] [PubMed] [Google Scholar]
- 7.Goetghebuer T, Haelterman E, Le Chenadec J, et al. Effect of early antiretroviral therapy on the risk of AIDS/death in HIV-infected infants. AIDS. 2009;23:597–604. doi: 10.1097/QAD.0b013e328326ca37. [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization. Policy Brief: Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection. Geneva, Switzerland: 2015. pp. 1–67. [Google Scholar]
- 9.Children PoATaMMoH-I. [July 15 2015];Guidelines for the use of antiretroviral agents in pediatric HIV infection. Available at: http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 10.Dunn DT, Brandt CD, Krivine A, et al. The sensitivity of HIV-1 DNA polymerase chain reaction in the neonatal period and the relative contributions of intra-uterine and intra-partum transmission. AIDS. 1995;9:F7–F11. doi: 10.1097/00002030-199509000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Kovacs A, Xu J, Rasheed S, et al. Comparison of a rapid nonisotopic polymerase chain reaction assay with four commonly used methods for the early diagnosis of human immunodeficiency virus type 1 infection in neonates and children. Pediatr Infect Dis J. 1995;14:948–954. doi: 10.1097/00006454-199511000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Lilian R, Kalk E, Bhowan K, et al. Early diagnosis of in utero and intrapartum HIV infection in infants prior to 6-Weeks of age. J Clin Microbiol. 2012;50:2373–2377. doi: 10.1128/JCM.00431-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chatterjee A, Triapthi S, Gass R, et al. Implementing services for early infant diagnosis (EID) of HIV: a comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11:553. doi: 10.1186/1471-2458-11-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braun M, Kabue MM, McCollum ED, et al. Inadequate coordination of maternal and infant HIV services detrimentally affects early infant diagnosis outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shiau S, Arpadi S, Strehlau R, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162:1138–1145. 1145.e1–2. doi: 10.1016/j.jpeds.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coulibaly M, Meda N, Yonaba C, et al. Missed opportunities for early access to care of HIV-infected infants in Burkina Faso. PLoS One. 2014;9:e111240. doi: 10.1371/journal.pone.0111240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Innes S, Lazarus E, Otwombe K, et al. Early severe HIV disease precedes early antiretroviral therapy in infants: are we too late? J Int AIDS Soc. 2014;17:18914. doi: 10.7448/IAS.17.1.18914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aliyu MH, Blevins M, Megazzini KM, et al. Correlates of suboptimal entry into early infant diagnosis in rural north central Nigeria. J Acquir Immune Defic Syndr. 2014;67:e19–e26. doi: 10.1097/QAI.0000000000000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ciaranello AL, Park J-E, Ramirez-Avila L, et al. Early infant HIV-1 diagnosis programs in resource-limited settings: opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9:9–59. doi: 10.1186/1741-7015-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lilian RR, Kalk E, Technau KG, et al. Birth diagnosis of HIV infection on infants to reduce infant mortality and monitor for elimination of mother-to-child transmission. Pediatr Infect Dis J. 2013;32:1080–1085. doi: 10.1097/INF.0b013e318290622e. [DOI] [PubMed] [Google Scholar]
- 21.Mayaux M, Burgard M, Teglas J, et al. Neonatal Characteristics in Rapidly Progressive Perinatally Acquired HIV-1 Disease. JAMA. 1996;275:606–610. [PubMed] [Google Scholar]
- 22.Montagu D, Yamey G, Visconti A, et al. Where do poor women in developing countries give birth? A multi-country analysis of demographic and health survey data. PLoS One. 2011;6:e17155. doi: 10.1371/journal.pone.0017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavarelli M, Scarlatti G. Human immunodeficiency virus type 1 mother-to-child transmission and prevention: successes and controversies. J Intern Med. 2011;270:561–579. doi: 10.1111/j.1365-2796.2011.02458.x. [DOI] [PubMed] [Google Scholar]
- 24.Chen JY, Ribaudo HJ, Souda S, et al. Highly active antiretroviral therapy and adverse birth outcomes among HIV-infected women in Botswana. J Infect Dis. 2012;206:1695–1705. doi: 10.1093/infdis/jis553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nielsen-Saines K, Watts D, Veloso VG, et al. Three postpartum antiretroviral regimens to prevent intrapartum HIV infection. N Engl J Med. 2012;366:2368–2379. doi: 10.1056/NEJMoa1108275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Burgard M, Blanche S, Jasseron C, et al. Performance of HIV-1 DNA or HIV-1 RNA tests for early diagnosis of perinatal HIV-1 infection during anti-retroviral prophylaxis. J Pediatr. 2012;160:60–66. e1. doi: 10.1016/j.jpeds.2011.06.053. [DOI] [PubMed] [Google Scholar]
- 27.Shapiro DE, Balasubramanian R, Fowler MG, et al. Time to HIV DNAPCR Positivity According to Maternal/infant Antiretroviral Prophylactic Regimen in Non-breastfed HIV-infected Infants in Populations with Predominantly Non-B HIV Subtype: A Collaborative Analysis of Data from Cohorts in Thailand, South Africa, Botswana and the United Kingdom [TUAB0203]; 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; July 17–20 2011; Rome, Italy. 2011. [Google Scholar]
- 28.Anoje C, Aiyenigba B, Suzuki C, et al. Reducing mother-to-child transmission of HIV: findings from an early infant diagnosis program in south-south region of Nigeria. BMC Public Health. 2012;12:1–8. doi: 10.1186/1471-2458-12-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao N-Y, Stinson K, Myer L. Linkage of HIV-infected infants from diagnosis to antiretroviral therapy services across the Western Cape, South Africa. PLoS One. 2013;8:e55308. doi: 10.1371/journal.pone.0055308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan AS, Sakwa EM, Nabwera HM, et al. Dynamics and constraints of early infant diagnosis of HIV infection in rural Kenya. AIDS Behav. 2012;16:5–12. doi: 10.1007/s10461-010-9877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Motswere-Chira C, Voetsch A, Lu L, et al. Follow-up of infants diagnosed with HIV — Early infant diagnosis program, Francistown, Botswana 2005–2012. MMWR Morb Mortal Wkly Rep. 2014;63:158–160. [PMC free article] [PubMed] [Google Scholar]
- 32.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: a randomized clinical trial. JAMA. 2000;283:1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 33.WHO. Global HIV/AIDS Response: Epidemic Update and Health Sector Progress Towards Universal Access. Geneva, Switzerland: World Health Organization; 2011. [Google Scholar]
- 34.UNAIDS Reference Group on Estimates, Modelling and Projections. Working paper on mother-to-child HIV transmission rates for use in spectrum. 2011:1–26. [Google Scholar]
- 35.Carter RJ, Dugan K, El-Sadr WM, et al. CD4+ cell count testing more effective than HIV disease clinical staging in identifying pregnant and postpartum women eligible for antiretroviral therapy in resource-limited settings. J Acquir Immune Defic Syndr. 2010;55:404–410. doi: 10.1097/QAI.0b013e3181e73f4b. [DOI] [PubMed] [Google Scholar]
- 36.Group KBS. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11:171–180. doi: 10.1016/S1473-3099(10)70288-7. [DOI] [PubMed] [Google Scholar]
- 37.WHO. Global and regional immunization profile. 2012;1 [Google Scholar]
- 38.Ekouevi DK, Kariyiare BG, Coffie PA, et al. Feasibility and acceptability of rapid HIV screening in a labour ward in Togo. J Int AIDS Soc. 2012;15:1–5. doi: 10.7448/IAS.15.2.17380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bello FA, Ogunbode OO, Adesina OA, et al. Acceptability of counselling and testing for HIV infection in women in labour at the university college hospital, Ibadan, Nigeria. Afr Health Sci. 2011;11:30–35. [PMC free article] [PubMed] [Google Scholar]
- 40.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]
- 41.Connolly MD, Rutstein RM, Lowenthal ED. Virologic testing in infants with perinatal exposure to HIV receiving multidrug prophylaxis. Pediatr Infect Dis J. 2013;32:196–197. doi: 10.1097/INF.0b013e3182787c29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiegert K, Dinh T-H, Mushavi A, et al. Integration of prevention of mother-to-child transmission of HIV (PMTCT) postpartum services with other HIV care and treatment services within the maternal and child health setting in Zimbabwe 2012. PLoS One. 2014;9:e98236. doi: 10.1371/journal.pone.0098236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wagner A, Slyker J, Langat A, et al. High mortality in HIV-infected children diagnosed in hospital underscores need for faster diagnostic turnaround time in prevention of mother-to-child transmission of HIV (PMTCT) programs. BMC Pediatr. 2015;15:1–7. doi: 10.1186/s12887-015-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fonjungo PN, Girma M, Melaku Z, et al. Field expansion of DNA polymerase chain reaction for early infant diagnosis of HIV-1: the Ethiopian experience. Afr J Lab Med. 2013;2:31. doi: 10.4102/ajlm.v2i1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tejiokem MC, Faye A, Penda IC, et al. Feasibility of early infant diagnosis of HIV in resource-limited settings: the ANRS 12140-PEDIACAM study in Cameroon. PLoS One. 2011;6:e21840. doi: 10.1371/journal.pone.0021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cook RE, Ciampa PJ, Sidat M, et al. Predictors of successful early infant diagnosis of HIV in a rural district hospital in Zambézia, Mozambique. J acquir immune defic syndr. 2011;56:e104–e109. doi: 10.1097/QAI.0b013e318207a535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens W, Erasmus L, Moloi M, et al. Performance of a novel human immunodeficiency virus (HIV) type 1 total nucleic acid-based real-time PCR assay using whole blood and dried blood spots for diagnosis of HIV in infants. J Clin Microbiol. 2008;46:3941–3945. doi: 10.1128/JCM.00754-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sundaram MLB. Identification Patient Loss Points from Testing to Treatment Initiation Among Infants Tested in Swaziland. Cape Town, South Africa. 5th IAS Conference on HIV Pathogenesis and Treatment; 2009. [Google Scholar]
- 49.Kellerman SE, Sugandhi N. Pediatric AIDS in the elimination agenda. PLoS Med. 2013;10:e1001503. doi: 10.1371/journal.pmed.1001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lilian RR, Johnson LF, Moolla H, et al. A mathematical model evaluating the timing of early diagnostic testing in HIV-exposed infants in South Africa. J Acquir Immune Defic Syndr. 2014;67:341–348. doi: 10.1097/QAI.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 51.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: a pooled analysis of individual data from sub-Saharan Africa. International Journal of Epidemiology. 2011;40:385–396. doi: 10.1093/ije/dyq255. [DOI] [PMC free article] [PubMed] [Google Scholar]


