Abstract
Stem cell transplantation and low-energy shock-wave therapy (LESWT) have emerged as potential and effective treatment protocols for diabetic erectile dysfunction. During the tracking of transplanted stem cells in diabetic erectile dysfunction models, the number of visible stem cells was rather low and decreased quickly. LESWT could recruit endogenous stem cells to the cavernous body and improve the microenvironment in diabetic cavernous tissue. Thus, we deduced that LESWT might benefit transplanted stem cell survival and improve the effects of stem cell transplantation. In this research, 42 streptozotocin-induced diabetic rats were randomized into four groups: the diabetic group (n = 6), the LESWT group (n = 6), the bone marrow-derived mesenchymal stem cell (BMSC) transplantation group (n = 15), and the combination of LESWT and BMSC transplantation group (n = 15). One and three days after BMSC transplantation, three rats were randomly chosen to observe the survival numbers of BMSCs in the cavernous body. Four weeks after BMSC transplantation, the following parameters were assessed: the surviving number of transplanted BMSCs in the cavernous tissue, erectile function, real-time polymerase chain reaction, and penile immunohistochemical assessment. Our research found that LESWT favored the survival of transplanted BMSCs in the cavernous body, which might be related to increased stromal cell-derived factor-1 expression and the enhancement of angiogenesis in the diabetic cavernous tissue. The combination of LESWT and BMSC transplantation could improve the erectile function of diabetic erectile function rats more effectively than LESWT or BMSC transplantation performed alone.
Keywords: bone marrow mesenchymal stem cell, diabetes mellitus, erectile function, low-energy shock-wave therapy
INTRODUCTION
Erectile dysfunction (ED) has been defined as the persistent inability to attain and maintain an erection sufficient to permit satisfactory sexual performance. ED affects physical and psychological health and has a significant impact on the life quality of sufferers and their partners.1 ED is one of the major complications of diabetes mellitus (DM).2 The pathogenesis of diabetic ED is multifactorial and complicated, involving persistent damage to the vascular endothelium and smooth muscle and cavernous fibrosis.3,4
Today, oral phosphodiesterase-5 inhibitors (PDE5i) are generally considered the first-line choice for ED patients. However, approximately 35% of patients with ED still fail to respond to this treatment. In these patients, DM is one of the most common causes of failure to respond.5 In diabetic men, the efficacy of PDE5i is considerably <70%.6 For the purpose of exploring more effective therapeutic strategies for restoration of the diabetic cavernous body, regenerative medicine is an inevitable subject. Recently, stem cell transplantation and low-energy shock-wave therapy (LESWT) have emerged as potentially effective treatment protocols for diabetic ED.7,8
Bone marrow mesenchymal stem cells (BMSCs) are among the common stem cells transplanted in diabetic ED rats. These cells could improve erectile function, increase the content of endothelium and smooth muscle, and exert anti-fibrotic effects.9,10,11 On tracking transplanted stem cells in diabetic ED rat models, the number of visible BMSCs was rather low and decreased rapidly in the subsequent period.9 Similar results were achieved with adipose-derived mesenchymal stem cell transplantation in diabetic rats12 and different types stem cell transplantation in rat models of cavernous nerve injury.13 The low survival number of intracavernosally injected stem cells limited the therapeutic effects of BMSC transplantation.
The small visible number of transplanted stem cells might be related to the migration of intracavernosally injected stem cells because research has confirmed that intracavernosally injected stem cells exited the penis within days and migrated preferentially to the bone marrow.14 In addition, the poor viability of transplanted BMSCs might be related to the hostile survival microenvironment.15 The vasculature plays an instructive role in the stem cell niche16 whereas a growing body of evidence has indicated that diabetes impacts angiogenesis through a variety of mechanisms, including vascular endothelial growth factor (VEGF)/phosphoinositide 3’ kinase/AKT/endothelial nitric oxide synthase (eNOS) proangiogenic pathway abrogation and vessel (CD31+) number reduction.17,18
LESWT, which is typically defined as an energy flux density <0.1 mJ mm−2, can improve the erectile function of diabetic rats by promoting the regeneration of endothelial cells, smooth muscle cells, and exerting anti-fibrotic effects.19,20,21 In addition to improving angiogenesis, the mechanism of action of LESWT might be related to the recruitment of endogenetic stem cells.22 Intraperitoneally injected EdU-labeled mesenchymal stem cells (MSCs) are recruited by LESWT and migrate to the cavernous body.19
If LESWT can inhibit the migration of intracavernosally injected stem cells leaving the cavernous body and create a beneficial microenvironment for an exogenous stem cell niche to be established in the diabetic cavernous body, the survival of transplanted BMSCs would increase. Naturally, the combination of LESWT and BMSC transplantation should improve the erectile function of diabetic rats more effectively.
MATERIALS AND METHODS
Study design
Forty-eight diabetic rats were randomized into four groups. The DM group (n = 6) was diabetic control group. In the LESWT group (n = 6), the rats received a course of LESWT for 3 weeks. In the LESWT + BMSC group (n = 15), the rats received BMSC transplantation 1 day after 3-week course of LESWT. In the BMSC group (n = 15), the rats only received BMSC transplantation at the same time as the LESWT + BMSC group but without LESWT. Another six healthy adult rats that did not receive streptozotocin injections were included as normal group (normal group).
Before and at the end of the 3-week course of LESWT, the circulating endothelial progenitor cell (EPC) markers (CD31, CD34) of the groups (n = 12) that did or did not receive LESWT were evaluated by flow cytometry. At 1 day and 3 days after transplantation, three rats were chosen randomly from both BMSC group and LESWT + BMSC group separately to observe the number of labeled BMSCs. Four weeks after BMSC transplantation, intracavernous pressure (ICP)/mean arterial pressure (MAP) measurements, reverse transcription-polymerase chain reaction (RT-PCR) of stromal cell-derived factor-1 (SDF-1), and VEGF and penile histological assessment were performed.
Establishment of diabetic rat model
The establishment of the diabetic rat model was based on the process described before.9 Healthy adult male Sprague-Dawley rats (about 200 g and 8 weeks old) were intraperitoneally injected with 1% streptozotocin solution (65 mg kg−1). Diabetes was confirmed by measuring tail vein random blood glucose levels 72 h after injection. Rats with random blood glucose concentrations >16.7 mmol l−1 were diagnosed as diabetic. Random blood glucose from the tail vein blood was measured using a blood glucose meter (Roche, Basel, Switzerland) every week. The weight was also measured every week. The protocols were approved by the Committee of Ethics in Animal Experimentation of Southern Medical University. All the rats were maintained in a conventional temperature-controlled animal house with a 12 h light-dark cycle and with a continuous supply of food and water.
BMSC isolation culture, labeling, and cavernous injection
Isolation and expansion of BMSCs were performed according to a previous description.9 Male Sprague-Dawley rats (4 weeks old) were sacrificed after anesthesia, and bone marrow was harvested by flushing the femoral and tibial cavities with phosphate-buffered saline (PBS). The collected cells were seeded in culture medium at a density of 1 × 106 cells per ml Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum, 100 U ml−1 penicillin and 100 mg ml−1 streptomycin in culture flasks. The cells were incubated in a humidified atmosphere containing 5% CO2 at 37°C. Two days later, nonadherent cells were removed and fresh culture medium was added. The culture medium was changed every 3 days. Cells were passed when they reached approximately 90% confluence. The third-passage BMSC phenotype was identified by flow cytometry analysis.
The cultured third-passage BMSCs were labeled with the green fluorescent lipophilic dye cell marker-DiO (CM-DiO Vybrant™, Berkshire, UK) for cell tracking, according to the manufacturer's instructions. After labeling, BMSCs were suspended in PBS at a concentration of 2000 cells per μl for intracavernosal transplantation.
One day after 3-week course of LESWT, 1 × 106 BMSCs dissolved in 500 μl of PBS were injected into the cavernous bodies of the rats in the BMSC and LESWT + BMSC groups. Rats in the DM and LESWT groups received an injection of 500 μl of PBS.
LESWT treatment
LESWT was modified and performed according to previously published studies.19 The rats were anesthetized with pentobarbital. The penis was drawn out of the prepuce, and the ultrasonic gel was applied to the penis. Then, the penis was placed on a shock-wave applicator (Shenzhen Hyde Medical Equipment Co., Ltd., Shenzhen, China). A total of 300 shocks were delivered at an energy level of 11.08 MPa (approximately 0.082 mJ mm−2) at a frequency of 60 per min. The rats from the LESWT and LESWT + BMSC groups received LESWT treatment three times weekly for 2 weeks with a 1-week interval.
Flow cytometry for BMSC identification and circulating EPC counting
Passage three BMSCs were prepared for flow cytometry analysis. In brief, 1 × 105 BMSCs were harvested and suspended in 500 μl of PBS followed by incubation with monoclonal mouse IgG to CD45 (eBioscience, San Diego, CA, USA), monoclonal mouse IgG to CD34 (Santa Cruz Biotechnology, Inc., Dallas, TX, USA), monoclonal mouse IgG to CD44 (eBioscience, San Diego, CA, USA), monoclonal Armenian hamster IgG to CD29 (Biolegend, San Diego, CA, USA) and monoclonal mouse IgG to CD90 (Biolegend, San Diego, CA, USA). After washing with PBS, the BMSCs were analyzed using the FACS Fortessa (BD Biosciences, Franklin Lakes, NJ, USA).
Before and at the end of the 3-week course of LESWT, peripheral blood samples from the tail veins of the groups receiving LESWT (LESWT and LESWT + BMSC groups, n = 12) or not (DM and BMSC groups, n = 12) were obtained to detect CD31 (eBioscience, San Diego, CA, USA) and CD34 expression levels. The results were expressed as the percentage of the total mononuclear cell number.
Evaluation of erectile function
Four weeks after intracavernous transplantation of BMSCs, ICP/MAP response to electric stimulation of the cavernous nerve was measured as previously described.9 After administration of pentobarbital for anesthesia, a lower abdominal midline incision was performed and the cavernous nerve was exposed. A bipolar electrode connected to an electrical pulse stimulator was connected to the cavernous nerves. A 23-gauge needle connected to a tube filled with heparin was inserted into the penis crus for the measurement of ICP. The carotid artery was cannulated to record the MAP. The nerve was stimulated at a frequency of 10 Hz with a pulse width of 5 ms. Stimulation was performed at 10 V for 60 s with resting periods of 5 min between subsequent stimulations. Data were acquired using an MP150 physiological recorder (BIOPAC, Goleta, CA, USA).
RT-PCR of SDF-1 and VEGF expression
After erectile function evaluation, the rats were sacrificed and their penises were harvested. Part of the penis was chosen randomly for RNA isolation. Snap-frozen tissue was pulverized and then homogenized in TriZol (Invitrogen, Cergy-Pontoise, France). Primers were designed using Primer Premier software, version 5.0 (Primer, Palo Alto, CA, USA), and their sequences are listed as follows. VEGF primers: upstream primer 5’-CAATGATGAAGCCCTGGAGT-3’, downstream primer 5’- TATGTGCTGGCTTTGGTGAG-3’; and SDF-1 primers: upstream primer 5’-GCTCTGCATCAGTGACGGTA-3’, downstream primer 5’- GATGTTTGACGTTGGCTCTG-3’.
Reverse transcription was performed in strict accordance with the TaKaRa PrimeScript II 1st Strand cDNA short Kit (TaKaRa Bio Inc., Dalian, China) instructions. RT-PCR was performed according to the TaKaRa SYBR® Premix Ex Taq™ II and Bio-Rad CFX96 Real-Time PCR System operating directions (Bio-Rad, Hercules, CA, USA). Data analysis was performed with Bio-Rad CFX Manager software, version 1.6 (Bio-Rad, Hercules, CA, USA). Expression of the gene of interest was computed relative to the expression of the internal standard β-actin.
Fluorescence microscopy and immunohistochemistry
At 1 day and 3 days after BMSC transplantation, freshly dissected tissues of the cavernous bodies of three rats from the LESWT + BMSC and BMSC groups were harvested to evaluate the numbers of transplanted BMSCs. Four weeks after transplantation and immediately after ICP/MAP evaluation, part of the freshly dissected tissue from the remainder of the cavernous bodies was harvested to evaluate the transplanted BMSC survival numbers. The samples were fixed and detected by fluorescence microscopy. We randomly chose five slices from each cavernous body for calculations.
Tissue from every group was fixed in 4% paraformaldehyde in PBS overnight, dehydrated by an ethanol gradient, and then embedded in paraffin. Paraffin-embedded sections 5 μm in size were prepared. The tissue was immune-stained with anti-α-smooth muscle actin (α-SMA; Bioworld, Saint Louis Park, MN, USA), anti-CD31 (Bioworld, Saint Louis Park, MN, USA), or anti-eNOS (Bioworld, Saint Louis Park, MN, USA). Masson Trichrome staining was performed and expressed as the ratio of smooth muscle/collagen. Images with ×200 magnification were obtained.
For tissue specimens, data were averaged from six fields of each section. Images were obtained using an Olympus BX51 microscope (Olympus, Tokyo, Japan) and an Olympus DP71 camera (Olympus, Tokyo, Japan). Quantitative image analysis was conducted through computerized densitometry utilizing the ImagePro computer program version 6.0 (Media Cybernetics, Rockville, MD, USA). The resulting data were expressed as the ratio of the average stained area relative to the DM group.
Statistical analysis
The outcomes are shown as the mean ± standard error. Statistical comparisons among the groups were performed with one-way ANOVA. The unpaired t-test was performed between two groups using SPSS software, version 17.0 (SPSS, Chicago, IL, USA).
RESULTS
Characterization of BMSCs
After three passages, the BMSCs were identified by flow cytometry analysis, demonstrating that cultured cells positively expressed CD44, CD90, and CD29 but failed to express CD34 and CD45. These results indicate that the cultured cells were of mesenchymal origin with high purity (Figure 1).
Figure 1.
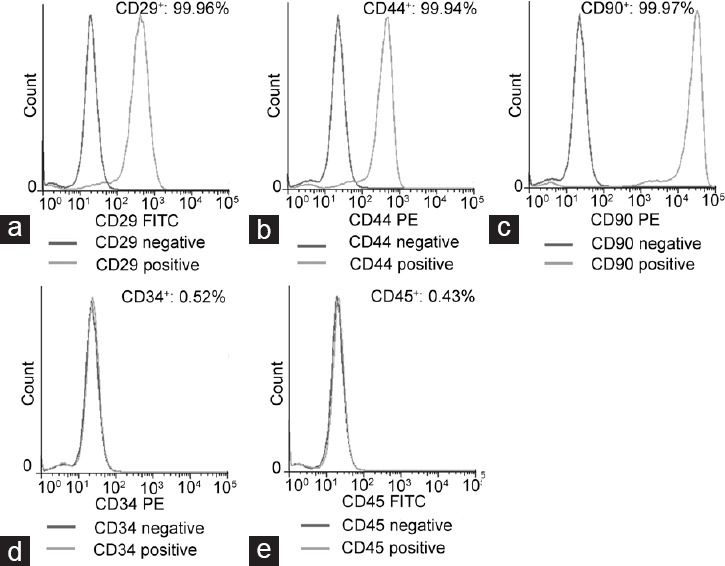
Flow cytometric identification of BMSCs: flow cytometric analysis revealed that cultured BMSCs highly expressed CD29 (a), CD44 (b), and CD90 (c) but did not express CD34 (d) and CD45 (e). BMSC: bone marrow mesenchymal stem cell; PE: phycoerythrin; FITC: fluorescein isothiocyanate.
CD31/CD34 cell marker expression
Before LESWT, no difference in the number of circulating CD31/CD34-positive cells (expressed as percentage) was noted between the rats receiving LESWT and those that did not (rats with LESWT vs rats without LESWT: 5.93 ± 0.66 vs 6.66 ± 0.88, P > 0.05). At the end of the 3-week course of LESWT, the number of circulating CD31/CD34-positive cells (expressed as percentage) in the rats that received LESWT increased compared with the rats that did not receive LESWT (rats with LESWT vs rats without LESWT: 13.59 ± 1.38 vs 4.03 ± 0.98, P < 0.001; Figure 2).
Figure 2.
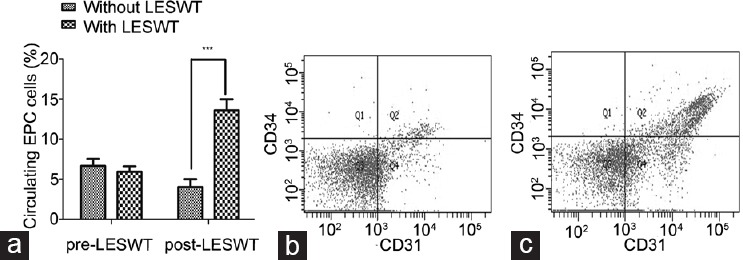
Flow cytometric analysis of circulating EPCs. (a) Impact of LESWT on percentage of circulating EPC. (b) Percentage of circulating EPC of rats without LESWT. (c) Percentage of circulating EPC of rats with LESWT. Flow cytometric analysis of peripheral blood revealed significantly increased number of circulating CD31/CD34-positive endothelial progenitor cells at the end of a 3-week course of LESWT (***P < 0.001). EPC: endothelial progenitor cell; LESWT: low-energy shock-wave therapy.
Survival of transplanted BMSCs in cavernous bodies
One day after BMSC transplantation, the numbers of stem cells implanted in the cavernous bodies in both the BMSC group and the LESWT + BMSC group were similar (BMSC group vs LESWT + BMSC group: 30.13 ± 2.25 vs 34.53 ± 1.85, P > 0.05, per ×200 magnification). Three days after transplantation, in the BMSC group, the stem cells disappeared quickly. However, in the LESWT + BMSC group, most of the stem cells remained in the cavernous body (BMSC group vs LESWT + BMSC group: 4.00 ± 0.60 vs 23.00 ± 2.02, P < 0.001, per × 200 magnification). Four weeks after BMSC transplantation, almost no CM-DiO labeled BMSCs were identified in the cavernous body per field (0.20 ± 0.07, per ×200 magnification) in the BMSC group. In contrast, in the LESWT + BMSC group, the number of CM-DiO labeled BMSCs in the specimens per field were increased approximately 10-fold (7.63 ± 0.58 per ×200 magnification, P < 0.001; Figure 3).
Figure 3.
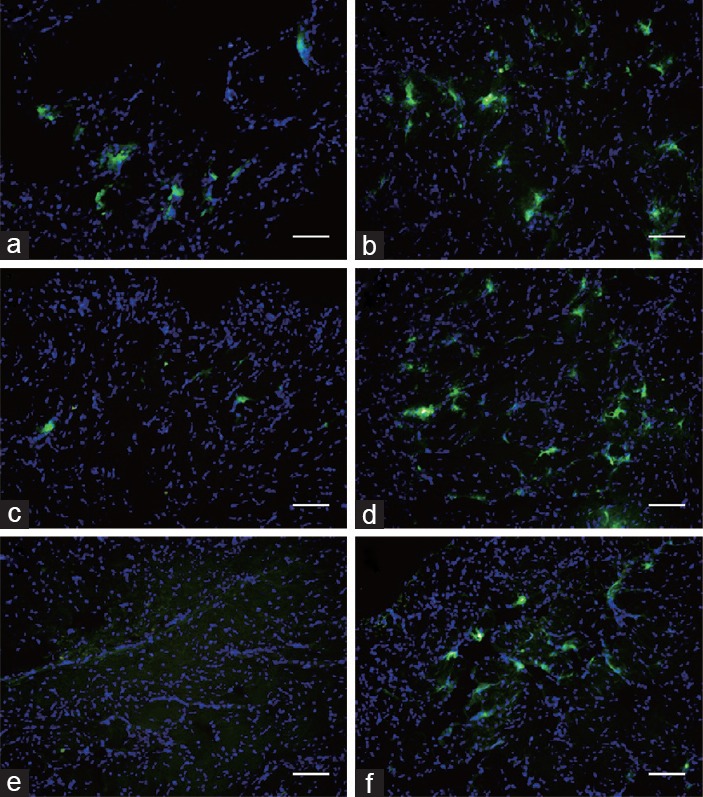
Survival of transplanted BMSCs in cavernous tissue: green fluorescent light CM-DiO labeled BMSCs implanted in the cavernous body were similar in the BMSC and LESWT + BMSC groups 1 day after transplantation. The stem cells in the BMSC group disappeared very quickly, and almost no transplanted stem cells remained at the end. Three days after transplantation, the number of CM-DiO-labeled BMSCs that survived in the cavernous body exhibited minimal change in the LESWT + BMSC group. Four weeks after BMSC transplantation, more CM-DiO labeled BMSCs could be observed in the LESWT + BMSC group compared with the BMSC group. (a and b) One day after transplantation. (c and d) Three days after transplantation. (e and f) Four weeks after transplantation. (a, c and e) BMSC group. (b, d and f) LESWT + BMSC group. Scale bar: 75 µm. BMSC: bone marrow mesenchymal stem cell; LESWT: low-energy shock-wave therapy; CM-DiO: cell marker-DiO.
Erectile function
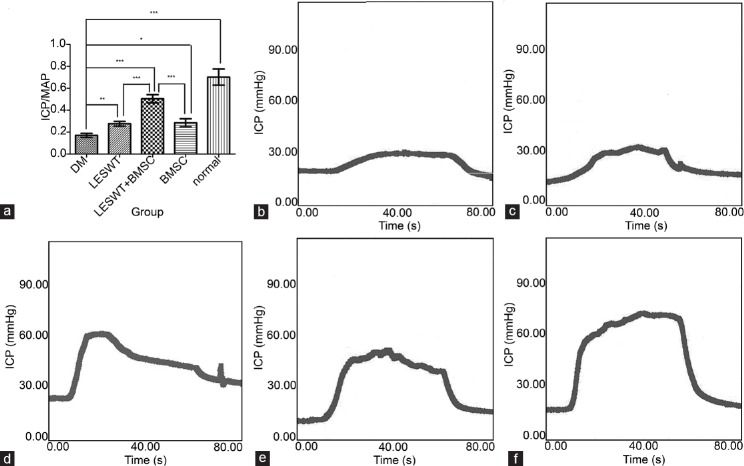
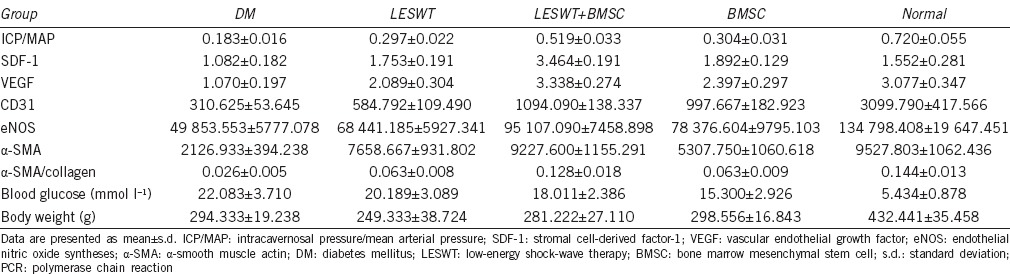
Four weeks after transplantation, the mean of ICP/MAP in the DM group was reduced compared with the normal group. Both LESWT and BMSC transplantation could improve the ICP/MAP ratio whereas the combination of LESWT and BMSC transplantation improved the ICP/MAP ratio more effectively than LESWT or BMSC transplantation performed alone (Figure 4 and Table 1).
Figure 4.
Measurement of ICP/MAP. Both LESWT and BMSC transplantation significantly improved the ICP/MAP of diabetic rats. The combination of LESWT and BMSC transplantation was more effective in improving the erectile function of diabetic rats than LESWT or BMSC transplantation alone. (a) Results of erectile function expressed as the ratio of ICP/MAP. (b) DM group. (c) LESWT group. (d) LESWT + BMSC group. (e) BMSC group. (f) Normal group. *P < 0.05; **P < 0.01; ***P < 0.001. ICP: intracavernosal pressure; MAP: mean arterial pressure; LESWT: low-energy shock-wave therapy; BMSC: bone marrow mesenchymal stem cell; DM: diabetes mellitus.
Table 1.
Results of erectile function, PCR, immunohistochemistry, blood glucose and body weight
RT-PCR of SDF-1 and VEGF expression
Both LESWT and BMSC transplantation improve SDF-1 and VEGF expression. The combination of LESWT and BMSC transplantation improved SDF-1 and VEGF expression more than LESWT or BMSC transplantation alone (Figure 5 and Table 1).
Figure 5.
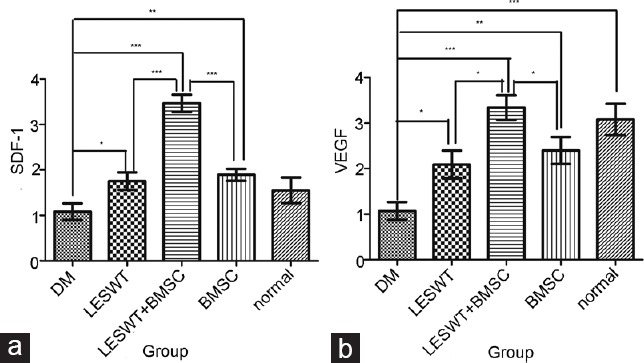
SDF-1 and VEGF expression in the cavernous tissue. SDF-1 and VEGF gene expression in the LESWT group, BMSC group and LESWT + BMSC group was significantly increased compared with the DM group, whereas increased SDF-1 and VEGF expression in the LESWT + BMSC group was more remarkable. (a) SDF-1 expression in the cavernous tissue. (b) VEGF expression in the cavernous tissue. *P < 0.05; **P < 0.01; ***P < 0.001. SDF-1: stromal cell-derived factor-1; VEGF: vascular endothelial growth factor; LESWT: low-energy shock-wave therapy; BMSC: bone marrow mesenchymal stem cell; DM: diabetes mellitus.
Endothelial marker (CD31) expression in cavernous tissues
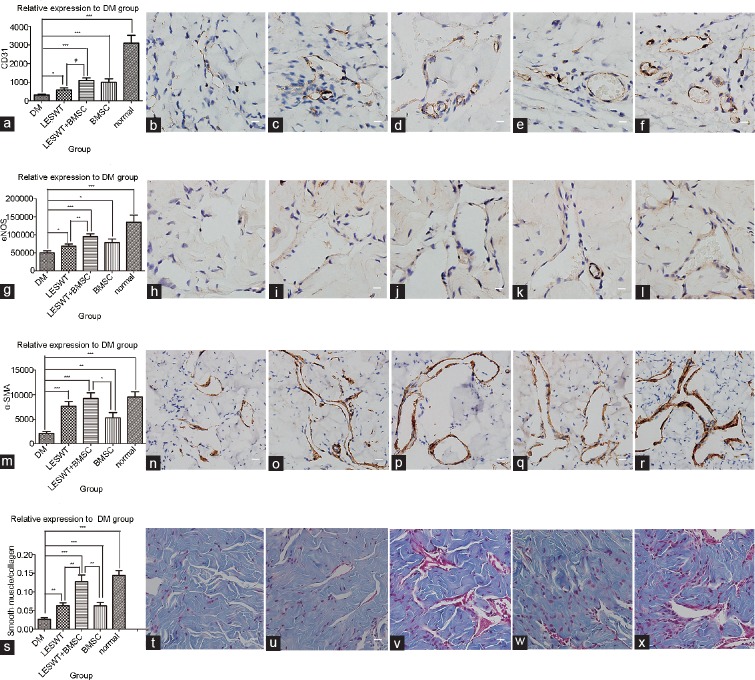
To understand the impacts of different treatments on angiogenesis, immunohistochemical analysis was performed using the vascular endothelial marker CD31. Positive areas of CD31 expression were considerably reduced within the cavernous bodies of the diabetic rats compared with normal rats. Four weeks after LESWT, improvement in CD31 expression was noted in both the LESWT group and the BMSC group. The combination of LESWT and BMSC transplantation achieved better effects than LESWT alone (Figure 6a–6f and Table 1).
Figure 6.
Immunohistochemistry of the cavernous tissue. Brown dye indicated the target factor-positive expression area. CD31, eNOS, or α-SMA-positive stained cells identified in the cavernous body appeared mostly on the inner wall surface of the vasculature or inside the vessel walls. The average stained area was expressed relative to the DM group. Compared with the normal group, a significant decrease in the positively stained area of CD31, eNOS, α-SMA or smooth muscle/collagen ratio was observed in the DM group, suggesting serious cell damage and fibrosis in penile tissue. After diabetic rats were treated by LESWT or BMSC transplantation, CD31-, eNOS-, and α-SMA-positive stained areas and smooth muscle/collagen ratio increased significantly. The combination of LESWT and BMSC transplantation increased CD31 and eNOS expression more effectively than LESWT, increased α-SMA expression more effectively than BMSC transplantation, and increased the smooth muscle/collagen ratio more effectively than LESWT and BMSC transplantation performed alone. (a–f) CD31 expression in the cavernous tissue. (g–l) eNOS expression in the cavernous tissue. (m–r) α-SMA expression in the cavernous tissue. (s–x) Smooth muscle/collagen ratio in the cavernous tissue. (a, g, m and s) Cell markers relative expression to DM group. (b, h, n and t) DM group. (c, i, o and u) LESWT group. (d, j, p and v) LESWT + BMSC group. (e, k, q and w) BMSC group. (f, l, r and x) Normal group. *P < 0.05; **P < 0.01; ***P < 0.001. Scale bar: 25 µm. eNOS: endothelial nitric oxide synthase; α-SMA: α-smooth muscle actin; DM: diabetes mellitus; LESWT: low-energy shock-wave therapy; BMSC: bone marrow mesenchymal stem cell.
eNOS expression in cavernous tissues
To understand the impacts of different treatments on endothelial functions, immunohistochemical analysis was performed using eNOS. The positive area of eNOS expressed within the cavernous tissue significantly decreased in the DM group compared with the normal group. eNOS expression in the LESWT group or the BMSC group was increased compared with the DM group. The combination of LESWT and BMSC transplantation was more effective than LESWT alone (Figure 6g–6l and Table 1).
α-SMA expression in cavernous tissues
Immunohistochemical analysis was performed using α-SMA to reveal the impacts of different treatments on muscle restoration. Compared with the normal group, α-SMA expression in the cavernous bodies of the diabetic group was reduced. Both LESWT and BMSC transplantation could increase α-SMA expression levels, but the combination of LESWT and BMSC transplantation increased α-SMA expression levels more than BMSC transplantation alone (Figure 6m–6r and Table 1).
Masson's trichrome staining of cavernous tissues
The fibrosis degree was evaluated by Masson's trichrome staining. The smooth muscle/collagen ratio in the DM group was reduced compared with the normal group. The smooth muscle/collagen ratio was increased significantly in both the LESWT group and the BMSC group, whereas the LESWT + BMSC group outperformed the LESWT and BMSC groups (Figure 6s–6x and Table 1).
Serum glucose and body weight
Four weeks after BMSC transplantation, no remarkable differences in blood glucose or body weight were noted among the four diabetic groups (Table 1).
DISCUSSION
BMSC transplantation holds great promise for the future of regenerative medicine for diabetic ED due to its multilineage differentiation and self-renewal capacity.9,10,11,23,24 The low BMSC survival number in the cavernous body after transplantation caused paracrine to be the main mechanism of transplanted BMSCs used currently.9,23 Using a combination of LESWT and BMSC transplantation, we recruited more transplanted BMSCs to the cavernous body and improved the erectile function of diabetic rats more effectively than BMSC transplantation alone. We believe that the mechanism of action might be related to increased SDF-1 expression and enhanced angiogenesis in the cavernous body through LESWT.
One characteristic of BMSC migration is that it occurs against the blood concentration gradient of SDF-1.25 In our research, SDF-1 gene expression in rats receiving LESWT was increased compared with rats that did not receive LESWT, indicating that the SDF-1 expression level in the cavernous tissue was increased by LESWT. In early stages (3 days) after BMSC transplantation, more labeled BMSCs were noted in the LESWT + BMSC group, indicating that the migration of exogenous BMSCs was inhibited by the high level of SDF-1 expression in the cavernous body induced by LESWT. LESWT improved SDF-1 expression levels, and SDF-1 mediated the migration of BMSCs, as demonstrated in hind limb ischemia rats, chronic spinal cord injury rats, acute myocardial infarction pig models,26,27,28 heart myocardial infarction rats and liver failure mouse models.29,30
MSCs were located around the capillaries in a variety of tissues,31 and the vasculature played an instructive role in the stem cell niche;16 in particular, CD31+ cells, which proved to be EPCs, might be an important component of the BMSC niche.32 CD31 expressed on vascular endothelial cells has been considered an angiogenesis cell marker of diabetic ED rats.9,33,34 An endothelial marker (CD31) and a progenitor cell marker (CD34) were described to evaluate the effects of LESWT on the circulating EPC count.35 In streptozotocin-induced DM rats, the vasculature was impaired, and both cavernous CD31+ cells36 and circulating EPCs decreased dramatically.37,38,39 The reduction in circulating EPCs decreased the number of cells that could migrate to sites of damaged endothelium and differentiate into endothelial cells thereafter.40
In this research, SDF-1 and VEGF gene expression, circulating CD31/CD34-positive EPC numbers and the CD31+ expression in cavernous tissue were increased in the LESWT group compared with the DM group. Angiogenesis in the cavernous body induced by LESWT might be related to two sources of endogenous stem cell activation.41 On the one hand, both SDF-1 and VEGF could recruit endogenous EPCs,42,43 and LESWT could mobilize more EPCs enter in circulating and recruit to the cavernous tissue. On the other hand, LESWT could enhance stem cell proliferation, differentiation and paracrine.44,45,46,47 In addition, the types of stem cells detected around the cavernous vasculature, including perisinusoidal and subtunic penile progenitor cells48 and cavernous smooth muscle-derived stem cells,49,50 could be induced to activate. Thus, LESWT induced angiogenesis and created a beneficial situation for the establishment of the stem cell niche. Four weeks after BMSC transplantation, more surviving BMSCs were noted in the LESWT + BMSC group compared with the BMSC group.
Endothelial cell dysfunction reduces eNOS bioavailability. Smooth muscle loss and fibrosis caused by phenotype modulation51 and apoptosis52 reflect a decrease in the α-SMA or smooth muscle/collagen ratio. In our research, both LESWT and BMSC transplantation increase the CD31, eNOS, α-SMA and smooth muscle/collagen ratios of the diabetic rats, indicating that both of these treatments increase the content of endothelium and smooth muscle. The addition of LESWT could benefit implantation and increase the number of surviving transplanted BMSCs. The combination of LESWT and BMSC transplantation could enhance erectile function restoration more effectively. Similar conclusions have been achieved both in critical limb ischemia rats53 and myocardial infarction swine.54
In brief, LESWT could increase SDF-1 levels, which could subsequently inhibit the migration of transplanted BMSCs and facilitate the implantation of transplanted BMSCs in the cavernous body. LESWT could also enhance revascularization in the cavernous body, which might create beneficial conditions for transplanted BMSCs to survive. The combination of LESWT and BMSC transplantation was more effective in restoring erectile function in diabetic rats than LESWT or BMSC transplantation alone.
AUTHOR CONTRIBUTIONS
HTS presented the study design and participated in BMSC preparing, DM rat model building, LWSWT, FCM, ICP/MAP assessment, statistical analysis and drafted the manuscript. HBZ participated in BMSC preparing, ICP/MAP assessment, RT-PCR and immunohistochemical assessment. WTC participated in study design, supplied LESWT device and performed energy measurement. FZC participated in BMSC preparing, RT-PCR and immunohistochemical assessment. TW participated in study design and statistical analysis. JTL participated in ICP/MAP assessment. MY participated in DM rat model building. JHL participated in DM rat model building. AYW conceived of the study, and participated in its design and coordination and helped to draft. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was funded by grants from the National Natural Science Foundation of China (81170566) and the Natural Science Foundation of Guangdong Province (2014A030313302 and 2015A030310075).
REFERENCES
- 1.Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol. 2010;57:804–14. doi: 10.1016/j.eururo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 2.De Berardis G, Franciosi M, Belfiglio M, Di Nardo B, Greenfield S, et al. Erectile dysfunction and quality of life in type 2 diabetic patients: a serious problem too often overlooked. Diabetes Care. 2002;25:284–91. doi: 10.2337/diacare.25.2.284. [DOI] [PubMed] [Google Scholar]
- 3.Gratzke C, Angulo J, Chitaley K, Dai YT, Kim NN, et al. Anatomy, physiology, and pathophysiology of erectile dysfunction. J Sex Med. 2010;7:445–75. doi: 10.1111/j.1743-6109.2009.01624.x. [DOI] [PubMed] [Google Scholar]
- 4.Kovanecz I, Nolazco G, Ferrini MG, Toblli JE, Heydarkhan S, et al. Early onset of fibrosis within the arterial media in a rat model of type 2 diabetes mellitus with erectile dysfunction. BJU Int. 2009;103:1396–404. doi: 10.1111/j.1464-410X.2008.08251.x. [DOI] [PubMed] [Google Scholar]
- 5.Shamloul R, Ghanem H. Erectile dysfunction. Lancet. 2013;381:153–65. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 6.Hatzimouratidis K, Hatzichristou D. How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep. 2014;14:545. doi: 10.1007/s11892-014-0545-6. [DOI] [PubMed] [Google Scholar]
- 7.Lin CS, Xin Z, Dai J, Huang YC, Lue TF. Stem-cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2013;13:1585–97. doi: 10.1517/14712598.2013.847085. [DOI] [PubMed] [Google Scholar]
- 8.Lei H, Liu J, Li H, Wang L, Xu Y, et al. Low-intensity shock wave therapy and its application to erectile dysfunction. World J Mens Health. 2013;31:208–14. doi: 10.5534/wjmh.2013.31.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu X, Lin H, Wang Y, Yu W, Chen Y, et al. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 10.Qiu X, Sun C, Yu W, Lin H, Sun Z, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012;33:37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 11.Ryu JK, Kim DH, Song KM, Ryu DS, Kim SN, et al. Intracavernous delivery of clonal mesenchymal stem cells rescues erectile function in the streptozotocin-induced diabetic mouse. Andrology. 2016;4:172–84. doi: 10.1111/andr.12138. [DOI] [PubMed] [Google Scholar]
- 12.Nishimatsu H, Suzuki E, Kumano S, Nomiya A, Liu M, et al. Adrenomedullin mediates adipose tissue-derived stem cell-induced restoration of erectile function in diabetic rats. J Sex Med. 2012;9:482–93. doi: 10.1111/j.1743-6109.2011.02469.x. [DOI] [PubMed] [Google Scholar]
- 13.Shan H, Chen F, Zhang T, He S, Xu L, et al. Stem cell therapy for erectile dysfunction of cavernous nerve injury rats: a systematic review and meta-analysis. PLoS One. 2015;10:e121428. doi: 10.1371/journal.pone.0121428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin G, Qiu X, Fandel T, Banie L, Wang G, et al. Tracking intracavernously injected adipose-derived stem cells to bone marrow. Int J Impot Res. 2011;23:268–75. doi: 10.1038/ijir.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wan PX, Wang BW, Wang ZC. Importance of the stem cell microenvironment for ophthalmological cell-based therapy. World J Stem Cells. 2015;7:448–60. doi: 10.4252/wjsc.v7.i2.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Putnam AJ. The instructive role of the vasculature in stem cell niches. Biomater Sci. 2014;2:1562–73. doi: 10.1039/C4BM00200H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Howangyin KY, Silvestre JS. Diabetes mellitus and ischemic diseases: molecular mechanisms of vascular repair dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1126–35. doi: 10.1161/ATVBAHA.114.303090. [DOI] [PubMed] [Google Scholar]
- 18.Oviedo-Socarras T, Vasconcelos AC, Barbosa IX, Pereira NB, Campos PP, et al. Diabetes alters inflammation, angiogenesis, and fibrogenesis in intraperitoneal implants in rats. Microvasc Res. 2014;93:23–9. doi: 10.1016/j.mvr.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 19.Qiu X, Lin G, Xin Z, Ferretti L, Zhang H, et al. Effects of low-energy shockwave therapy on the erectile function and tissue of a diabetic rat model. J Sex Med. 2013;10:738–46. doi: 10.1111/jsm.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J, Zhou F, Li GY, Wang L, Li HX, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–73. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lei H, Xin H, Guan R, Xu Y, Li H, et al. Low-intensity pulsed ultrasound improves erectile function in streptozotocin-induced type I diabetic rats. Urology. 2015;86:1211–41. doi: 10.1016/j.urology.2015.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Abu-Ghanem Y, Kitrey ND, Gruenwald I, Appel B, Vardi Y. Penile low-intensity shock wave therapy: a promising novel modality for erectile dysfunction. Korean J Urol. 2014;55:295–9. doi: 10.4111/kju.2014.55.5.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun C, Lin H, Yu W, Li X, Chen Y, et al. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl. 2012;35:601–7. doi: 10.1111/j.1365-2605.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 24.He Y, He W, Qin G, Luo J, Xiao M. Transplantation KCNMA1 modified bone marrow-mesenchymal stem cell therapy for diabetes mellitus-induced erectile dysfunction. Andrologia. 2014;46:479–86. doi: 10.1111/and.12104. [DOI] [PubMed] [Google Scholar]
- 25.Won YW, Patel AN, Bull DA. Cell surface engineering to enhance mesenchymal stem cell migration toward an SDF-1 gradient. Biomaterials. 2014;35:5627–35. doi: 10.1016/j.biomaterials.2014.03.070. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Yan X, Wang C, Lu S, Tang T, et al. The effect of autologous endothelial progenitor cell transplantation combined with extracorporeal shock-wave therapy on ischemic skin flaps in rats. Cytotherapy. 2014;16:1098–109. doi: 10.1016/j.jcyt.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Ha KY, Kim JW, Seo JY, Kim YH. Does extracorporeal shock wave introduce alteration of microenvironment in cell therapy for chronic spinal cord injury? Spine (Phila Pa 1976) 2014;39:E1553–9. doi: 10.1097/BRS.0000000000000626. [DOI] [PubMed] [Google Scholar]
- 28.Lei PP, Tao SM, Shuai Q, Bao YX, Wang SW, et al. Extracorporeal cardiac shock wave therapy ameliorates myocardial fibrosis by decreasing the amount of fibrocytes after acute myocardial infarction in pigs. Coron Artery Dis. 2013;24:509–15. doi: 10.1097/MCA.0b013e3283640ec7. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Li M, Qu Z, Yan D, Li D, et al. SDF-1/CXCR4-mediated migration of transplanted bone marrow stromal cells toward areas of heart myocardial infarction through activation of PI3K/Akt. J Cardiovasc Pharmacol. 2010;55:496–505. doi: 10.1097/FJC.0b013e3181d7a384. [DOI] [PubMed] [Google Scholar]
- 30.Hao NB, Li CZ, Lu MH, Tang B, Wang SM, et al. SDF-1/CXCR4 axis promotes MSCs to repair liver injury partially through trans-differentiation and fusion with hepatocytes. Stem Cells Int 2015. 2015:960387. doi: 10.1155/2015/960387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gomez-Gaviro MV, Lovell-Badge R, Fernandez-Aviles F, Lara-Pezzi E. The vascular stem cell niche. J Cardiovasc Transl Res. 2012;5:618–30. doi: 10.1007/s12265-012-9371-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H, Xian L, Lin Z, Yang C, Zhang M, et al. Endothelial progenitor cells as a possible component of stem cell niche to promote self-renewal of mesenchymal stem cells. Mol Cell Biochem. 2014;397:235–43. doi: 10.1007/s11010-014-2191-3. [DOI] [PubMed] [Google Scholar]
- 33.Liu G, Sun X, Bian J, Wu R, Guan X, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:e72790. doi: 10.1371/journal.pone.0072790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang X, Liu C, Li S, Xu Y, Chen P, et al. Hypoxia precondition promotes adipose-derived mesenchymal stem cells based repair of diabetic erectile dysfunction via augmenting angiogenesis and neuroprotection. PLoS One. 2015;10:e118951. doi: 10.1371/journal.pone.0118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tepekoylu C, Wang FS, Kozaryn R, Albrecht-Schgoer K, Theurl M, et al. Shock wave treatment induces angiogenesis and mobilizes endogenous CD31/CD34-positive endothelial cells in a hindlimb ischemia model: implications for angiogenesis and vasculogenesis. J Thorac Cardiovasc Surg. 2013;146:971–8. doi: 10.1016/j.jtcvs.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Burchardt T, Burchardt M, Karden J, Buttyan R, Shabsigh A, et al. Reduction of endothelial and smooth muscle density in the corpora cavernosa of the streptozotocin induced diabetic rat. J Urol. 2000;164:1807–11. [PubMed] [Google Scholar]
- 37.Oikawa A, Siragusa M, Quaini F, Mangialardi G, Katare RG, et al. Diabetes mellitus induces bone marrow microangiopathy. Arterioscler Thromb Vasc Biol. 2010;30:498–508. doi: 10.1161/ATVBAHA.109.200154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, et al. Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes. 2014;63:1353–65. doi: 10.2337/db13-0894. [DOI] [PubMed] [Google Scholar]
- 39.Ferraro F, Lymperi S, Mendez-Ferrer S, Saez B, Spencer JA, et al. Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci Transl Med. 2011;3:104ra101. doi: 10.1126/scitranslmed.3002191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maiorino MI, Bellastella G, Petrizzo M, Della VE, Orlando R, et al. Circulating endothelial progenitor cells in type 1 diabetic patients with erectile dysfunction. Endocrine. 2015;49:415–21. doi: 10.1007/s12020-014-0478-5. [DOI] [PubMed] [Google Scholar]
- 41.Xin ZC, Xu YD, Lin G, Lue TF, Guo YL. Recruiting endogenous stem cells: a novel therapeutic approach for erectile dysfunction. Asian J Androl. 2016;18:10–5. doi: 10.4103/1008-682X.150040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motabi IH, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2012;26:267–78. doi: 10.1016/j.blre.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Asahara T, Takahashi T, Masuda H, Kalka C, Chen D, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–72. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Y, Wang J, Wang M, Sun P, Chen J, et al. Activation of bone marrow-derived mesenchymal stromal cells-a new mechanism of defocused low-energy shock wave in regenerative medicine. Cytotherapy. 2013;15:1449–57. doi: 10.1016/j.jcyt.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Raabe O, Shell K, Goessl A, Crispens C, Delhasse Y, et al. Effect of extracorporeal shock wave on proliferation and differentiation of equine adipose tissue-derived mesenchymal stem cells in vitro. Am J Stem Cells. 2013;2:62–73. [PMC free article] [PubMed] [Google Scholar]
- 46.Yip HK, Chang LT, Sun CK, Youssef AA, Sheu JJ, et al. Shock wave therapy applied to rat bone marrow-derived mononuclear cells enhances formation of cells stained positive for CD31 and vascular endothelial growth factor. Circ J. 2008;72:150–6. doi: 10.1253/circj.72.150. [DOI] [PubMed] [Google Scholar]
- 47.Xu P, Gul-Uludag H, Ang WT, Yang X, Huang M, et al. Low-intensity pulsed ultrasound-mediated stimulation of hematopoietic stem/progenitor cell viability, proliferation and differentiation in vitro. Biotechnol Lett. 2012;34:1965–73. doi: 10.1007/s10529-012-0984-6. [DOI] [PubMed] [Google Scholar]
- 48.Lin G, Alwaal A, Zhang X, Wang J, Wang L, et al. Presence of stem/progenitor cells in the rat penis. Stem Cells Dev. 2015;24:264–70. doi: 10.1089/scd.2014.0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu LJ, Xue BX, Chen D, Gao J, Yang DR, et al. Isolation and passage of muscle-derived stem cells from the rat penile corpora cavernosa and induction of differentiation into smooth muscle cells. Cytotechnology. 2014;66:987–94. doi: 10.1007/s10616-013-9651-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu LJ, Xue BX, Shan YX, Chen D, Gao J, et al. In vivo determination of muscle-derived stem cells in rat corpus cavernosum. Genet Mol Res. 2015;14:9951–62. doi: 10.4238/2015.August.21.1. [DOI] [PubMed] [Google Scholar]
- 51.Wei AY, He SH, Zhao JF, liu Y, Liu Y, et al. Characterization of corpus cavernosum smooth muscle cell phenotype in diabetic rats with erectile dysfunction. Int J Impot Res. 2012;24:196–201. doi: 10.1038/ijir.2012.16. [DOI] [PubMed] [Google Scholar]
- 52.Park K, Ryu KS, Li WJ, Kim SW, Paick JS. Chronic treatment with a type 5 phosphodiesterase inhibitor suppresses apoptosis of corporal smooth muscle by potentiating Akt signalling in a rat model of diabetic erectile dysfunction. Eur Urol. 2008;53:1282–8. doi: 10.1016/j.eururo.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Yeh KH, Sheu JJ, Lin YC, Sun CK, Chang LT, et al. Benefit of combined extracorporeal shock wave and bone marrow-derived endothelial progenitor cells in protection against critical limb ischemia in rats. Crit Care Med. 2012;40:169–77. doi: 10.1097/CCM.0b013e31822d74d0. [DOI] [PubMed] [Google Scholar]
- 54.Sheu JJ, Lee FY, Yuen CM, Chen YL, Huang TH, et al. Combined therapy with shock wave and autologous bone marrow-derived mesenchymal stem cells alleviates left ventricular dysfunction and remodeling through inhibiting inflammatory stimuli, oxidative stress & enhancing angiogenesis in a swine myocardial infarction model. Int J Cardiol. 2015;193:69–83. doi: 10.1016/j.ijcard.2015.03.044. [DOI] [PubMed] [Google Scholar]