Abstract
This study aimed to explore the therapeutic effects of adipose-derived stem cells (ADSCs)-based microtissues (MTs) on erectile dysfunction (ED) in streptozotocin (STZ)-induced diabetic rats. Fifty-six 8-week-old Sprague-Dawley rats received intraperitoneal injection of STZ (60 mg kg−1), and 8 weeks later, the determined diabetic rats randomly received intracavernous (IC) injection of phosphate buffer solution (PBS), ADSCs, or MTs. Another eight normal rats equally got IC injection of PBS. MTs were generated with a hanging drop method, and the injected cells were tracked in ADSC- and MT-injected rats. Four weeks after the treatments, intracavernous pressure (ICP), histopathological changes in corpus cavernosum (CC), and functional proteins were measured. Rat cytokine antibody array was used to detect ADSCs or MTs lysate. The results showed that MTs expressed vascular endothelial growth factor (VEGF), nerve growth factor (NGF), and tumor necrosis factor-stimulated gene-6 (TSG-6). MTs injection had a higher retention than ADSCs injection and MTs treatment improved ICP, neuronal nitric oxide synthase (nNOS) expression, smooth muscle, and endothelial contents in diabetic rats, ameliorated local inflammation in CC better. Thus, our findings demonstrate that IC injection of MTs improves erectile function and histopathological changes in STZ-induced diabetic rats and appears to be more promising than traditional ADSCs. The underlying mechanisms involve increased cell retention accompanied with neuroprotection and anti-inflammatory behaviors of the paracrine factors.
Keywords: adipose-derived stem cells, diabetes, erectile dysfunction, microtissues, paracrine factors
INTRODUCTION
Erectile dysfunction (ED) is more prevalent in men with diabetes mellitus (DM). The morbidity rate of ED among diabetic men varies from 35% to 90% due to different population and ages.1,2 Aside from the heavy burden, diabetes mellitus induced erectile dysfunction (DMED), pathogenic manifestations involving endothelial injury, neuropathy, microvascular and fibrous-muscular alterations is usually more serious and difficult to treat than nondiabetic.3,4 The oxidation of low-density lipoproteins and overproduction of oxygen-free radicals induced by hyperglycemia may lead to smooth muscle dysfunction.5 Hyperglycemia can result in mitochondrial fragmentation and apoptosis of endothelial cells.6 Moreover, DMED is highly associated with nerve growth factor (NGF) deficiency and impaired vascular endothelial growth factor (VEGF) signaling.2,4 Phosphodiesterase type 5 inhibitor (PDE5i) was considered to be the first-line pharmacologic therapy for ED since 1998; however, PDE5i showed less efficacy in treating DMED.3
Recently, many experimental approaches for DMED have emerged, including insulin treatment,7 antioxidant therapy,8 low energy shock wave therapy,9 stem cells, and gene therapy.10,11 Among these strategies, stem cell-based therapy is considered to be promising due to its ability to recover functional cells and tissues. For choosing the candidate stem cells, adipose-derived stem cells (ADSCs) have been proposed as one of the most suitable types. Similar to bone marrow stem cells (BMSCs), ADSCs possess the ability of self-renewing and differentiation.12 ADSCs can be collected in abundant quantities and harvested by a minimally invasive procedure. In addition, the successful transplantation of allogeneic ADSCs indicated a low immunogenicity of the cells.13 A growing body of evidence suggested the usage of ADSCs in several ED models.14,15,16 Of interest, the benefits of ADSCs for ED appear to be related to growth factor and cytokine activity.14 However, the single-cell-based injection is known as low cellular survival which is necessary to long-term success. Adult stem cells might lose many properties in ordinary adherent culture.17 Numerous reports noticed that adult stem cells cultured as spheroids which were scaffold-free could increase the therapeutic potential.18,19 Our previous study showed that ADSCs-based microtissues (MTs) improved erectile function through neuroprotection in a rat model of cavernous nerve injury.20 In this study, the efficacy of ADSCs and ADSC-based MTs was compared for the treatment of ED in streptozotocin (STZ)-induced diabetic rats and histologic changes were examined.
MATERIALS AND METHODS
Animals
A total of 64 male Sprague-Dawley rats aged 8 weeks old (body weight: 270–310 g) were purchased from the Animal Center of Peking University. The Committee for Animal Care and Use of Peking University approved the experiments. After harvesting of paratesticular fat, 56 rats were fasted for 16 h, and then intraperitoneally injected with STZ (60 mg kg−1; Sigma-Aldrich, St. Louis, MO, USA). Blood glucose levels were measured at 72 h after STZ injection using a blood glucose meter (B. Braun, Melsungen, Germany). Of the 56 rats, 54 (96.43%) were diabetic with a fasting glucose concentration higher than 300 mg dl−1. An intraperitoneal injection of phosphate buffer solution (PBS) was administered to 8 rats as control group. At 8 weeks after STZ injection, apomorphine (100 μg kg−1; Sigma-Aldrich, St. Louis, MO, USA) was used to screen the diabetic rats.16 DMED was present in 48 of the 54 (88.89%) rats. The 48 DMED rats were numbered and randomly assigned to three groups: intracavernous (IC) injection of PBS (n = 8, the DM + PBS group), ADSCs (n = 20, the DM + ADSCs group), and MTs (n = 20, the DM + MTs group). The control group also received an IC injection of PBS. ADSCs were labeled with the chloromethylbenzamido derivative 1,19-dioctadecyl-3,3,39,39-tetramethylindocarbocyanine perchlorate (CM-Dil; Molecular Probes, Carlsbad, CA, USA) and tracked at days 1, 7, and 14 (n = 4) after injection. At day 28, rats in each group were examined for ED before the harvest of tissues.
ADSCs isolation and MTs generation
ADSCs were isolated from paratesticular fat and cultured as previous standardized method.21,22 In brief, all the animals underwent lower abdominal midline incision and bilateral resection of paratesticular adipose tissue. The adipose tissue was rinsed with PBS containing 1% Streptomycin and Penicillin, chopped into small pieces, and incubated in 0.075% collagenase type IA (Sigma-Aldrich, St. Louis, MO, USA) for 80 min. The top lipid part was removed and the liquid part was centrifuged at 1000 ×g at room temperature for 10 min. Then, the remaining cells were suspended in low glucose Dulbecco's modified Eagle's medium (DMEM, Hyclone, Logan, UT, USA) supplemented with 1% Streptomycin and Penicillin and 10% fetal bovine serum (FBS, HyClone, Logan, UT, USA). After filtered through a 100-μm cell strainer, the suspension was planted in a 10-cm dish and cultured at 37°C in 5% CO2. MTs were generated with a hanging drop method according to our previous study.20 When reaching approximately 80% confluence, ADSCs in each dish were centrifuged and resuspended in 1.2 ml DMEM. Then, ADSCs (1 × 104 cells in 30 μl) were dropped onto the cover of new dishes (40 drops per dish), 5 ml PBS was added to each dish, and the cover was carefully placed back on the dish. Dishes were kept at 37°C in 5% CO2 for 3 days.
ADSCs and MTs characteristics
Osteogenic and adipogenic differentiations were performed on both ADSCs and MTs. ADSCs and 3-day-old MTs were seeded in 6-well tissue culture plates and cultured in rat adipose-derived stem cell osteogenic or adipogenic differentiation medium (Cyagen, Santa Clara, CA, USA). We replaced the medium every 3 days with the differentiating time of 21 days, removed the differentiation medium, and rinsed the well with PBS. The cells were fixed with 4% formaldehyde solution for 30 min, and then stained with Alizarin Red or Oil Red O working solution for 30 min. After rinsing, the cells were visualized under light microscope (Leica, Heidelberg, Germany).
Cell lysate from ADSCs or MTs was mixed with biotinylated detection antibodies, and then incubated with a rat cytokine antibody array membrane (R&D systems, Minneapolis, MN, USA) which containing capture antibodies of 29 different target proteins. After washing, the membrane was exposed using chemiluminescent detection reagents.
IC injection
Under aseptic conditions, the determined DMED and normal rats were anesthetized with 10% chloral hydrate (300 mg kg−1). The penis was exposed in each group, and a 24-gauge needle was used to inject a total of 1 × 106 ADSCs or 100 MTs (1 × 104 ADSCs per MT) in 100 μl PBS or only 100 μl PBS into the corpus cavernosum (CC).20 An elastic band was applied to the base of the penis and the pressure was maintained for 2 min after the injection.
Measurement of erectile function
Erectile function was determined by intracavernosal pressure (ICP) and mean arterial pressure (MAP) 4 weeks postinjection. Under 10% chloral hydrate, the major pelvic ganglion (MPG) and cavernous nerves (CN) were exposed through a midline laparotomy. The penile was exposed by removing overlying skin and ischiocavernosus muscle. One of the 24-gauge needles that were connected to PE-50 tubes with heparinized saline (250 IU ml−1) was inserted into the left carotid to measure MAP. The other one was inserted into corpus cavernosum (CC) to measure ICP. PE-50 tubes were connected to the data acquisition system (MP150, BIOPAC Systems Inc., Goleta, CA, USA). The CNs were stimulated using a stainless steel bipolar hook electrode with the following parameters: 20 Hz, pulse width of 0.2 ms, 1.5 mA, for 50 s. The ratio of maximal ICP (mm Hg) to MAP (mm Hg) was calculated.
Immunofluorescence staining
For immunofluorescence (IF) staining, the penile tissue (midshaft portion) and entire major pelvic ganglia (MPG) were fixed in fresh 4% paraformaldehyde, and then were immersed in 30% sucrose in PBS overnight at 4°C.15,20 The fixed tissues and 3-day-old MTs were cryoembedded in optimal cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and cut into 5 μm sections before mounting on slides. MPGs were cut crosswise in the middle. After permeabilization and blocking, the slides were incubated with primary antibodies, including rabbit anti-neuronal nitric oxide synthase (nNOS, 1:200; Abcam, Cambridge, UK), rabbit anti-NGF (1:400; Abcam, Cambridge, UK), rabbit anti-VEGF (1:200; Abcam, Cambridge, UK), rabbit anti-α smooth muscle actin (α-SMA, 1:1000; Abcam, Cambridge, UK), rabbit anti-von Willebrand factor (vWF, 1:2000; Abcam, Cambridge, UK), rabbit anti-CD31 (1:400; Abcam, Cambridge, UK), rabbit anti-CD34 (1:200; Abcam, Cambridge, UK), rabbit anti-CD105 (1:400; Abcam, Cambridge, UK), and mouse anti-tumor necrosis factor-induced protein-6 (TSG-6, 1:200; Abcam, Cambridge, UK) at 4°C overnight. At room temperature, the sections were rinsed and incubated with Alexa Fluor-594 conjugated secondary antibodies (Invitrogen, Carlsbad, CA, USA). Nuclei were stained by 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen) for 5 min. For tracking the labeled cells, the slides of penile tissues were stained with DAPI only. Slides were visualized under a fluorescence microscope (Leica, Heidelberg, Germany).
Western blotting
Total proteins of rat penis, ADSCs, and 3-day-old MTs were extracted by mechanical homogenization in lysis buffer containing protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, MO, USA). Proteins were quantified using a bicinchoninic acid (BCA) protein assay kit (Thermo Scientific, Waltham, MA, USA). A volume of 20 μg sample of each protein was electroseparated in a 10% sodium dodecyl sulfate-polyacrylamide gel and transferred to a polyvinylidene fluoride membrane (Millipore Corporation, Bedford, MA, USA). For incubation, the primary antibodies were rabbit anti-NGF (1:400), rabbit anti-nuclear factor kappa-B (NF-κB, 1:2000), rabbit anti-VEGF (1:200), rabbit anti-α-SMA (1:2000), rabbit anti-nNOS (1:400), mouse anti-tumor necrosis factor-induced protein-6 (TSG-6, 1:200; all from Abcam, Cambridge, UK), and mouse anti-glyceraldehyde phosphate dehydrogenase (GAPDH, 1:10 000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). After incubation with the appropriate secondary antibody (1:2000; Santa Cruz Biotechnology), membranes were exposed using chemiluminescent detection reagents. Images were obtained with a C-digit machine (LI-COR Biosciences, Cambridge, UK).
Image and statistical analysis
Histomorphometric results were representative values (n = 5 per group) and analyses for slides were performed using Image-Pro Plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). Western blotting (WB) and rat cytokine array results were analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA). Statistical analyses were performed with the SPSS 19.0 (SPSS Inc., Chicago, IL, USA). Results were expressed as mean ± standard deviation (s.d.) and values of P < 0.05 were considered statistically different. Multiple comparisons between groups were done using one-way analysis of variance followed by a post hoc analysis with the Tukey–Kramer test while a comparison between two groups was performed by t-test.
RESULTS
Rats blood glucose and body weight
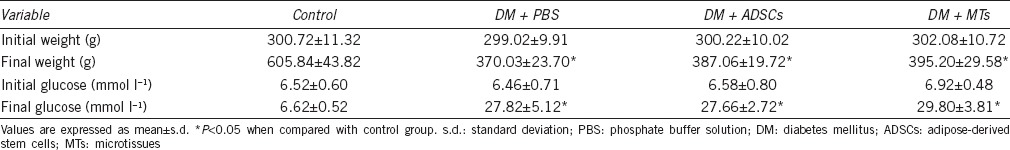
One rat in DM + PBS group and two rats in DM + ADSCs group were dead and abandoned during the experiment. The final blood glucose levels of STZ-induced diabetic rats were remarkably higher compared to PBS-treated control rats (P < 0.05). The final weights of diabetic rats were markedly lower than normal rats (P < 0.05). Among DM + PBS, DM + ADSCs, and DM + MTs groups, blood glucose levels and body weights were not statistically different (Table 1).
Table 1.
Body weight and blood glucose variables
Characterization of ADSCs and MTs
ADSCs cultured in a hanging drop gradually aggregated into a single central spheroid (Figure 1a), and the diameter of spheroid reduced around 175.11 ± 5.58 μm at the third day (Figure 1b). CM-Dil-labeled cells were showed in ADSCs or MTs. Immunofluorescence results showed that CD31 and CD34 were negative in MTs while CD105, vWF, NGF, VEGF, and TSG-6 were expressed in MTs (Figure 1c). Osteogenic and adipogenic differentiations demonstrated that both ADSCs and MTs have the potential for multipotency (Figure 1d).
Figure 1.
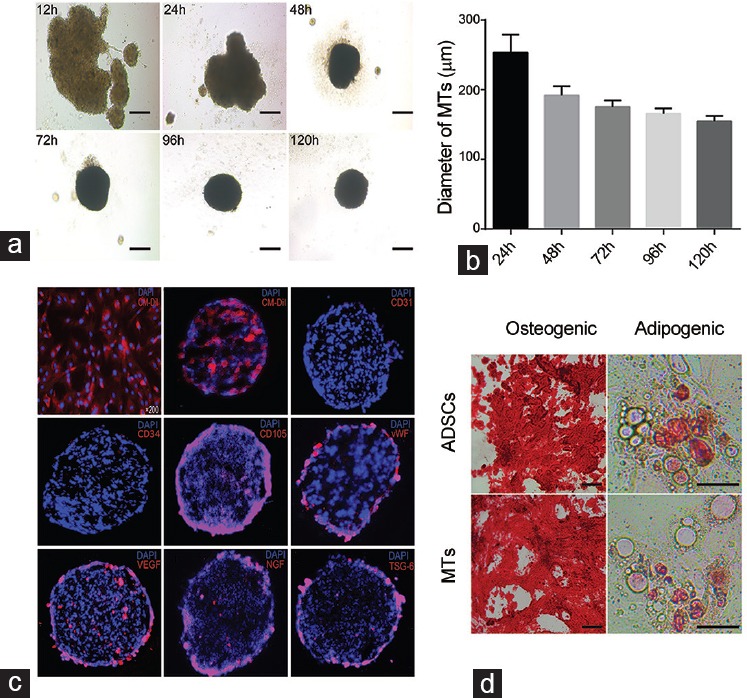
Characterization of the ADSCs and MTs. (a) Microscopy showing 10 000 ADSCs in a hanging drop gradually formed a stable spheroid with smooth edge and round shape. (b) The diameter of MTs was 253.67 ± 14.63 µm at 24 h, 191.90 ± 7.63 µm at 48 h, 175.12 ± 5.52 µm at 72 h, 165.66 ± 4.42 µm at 96 h, and 154.88 ± 4.15 µm at 120 h. Insert bar = 100 µm. (c) ADSCs and MTs were labeled with CM-Dil. MTs did not express CD31 and CD34 but expressed CD105, vWF, VEGF, NGF, and TSG-6. Original magnification: ×200. (d) Photomicrographs showing osteogenic (Alizarin Red staining) and adipogenic (Oil Red O staining) differentiations of ADSCs and MTs. Insert bar = 50 µm.
The disappearance of labeled cells in ADSCs- and MT-injected rats
The CM-Dil-labeled cells were tracked in ADSCs- and MT-injected rats at days 1, 7, and 14 post-IC injection (Figure 2a). As they were showed by integral optical density (IOD), cells disappeared rapidly in ADSC-injected rats compared with MT-injected rats (Figure 2b). At days 7 and 14, cells remained retaining in MT-injected rats while very few cells could be observed in ADSC-injected rats (P < 0.05).
Figure 2.
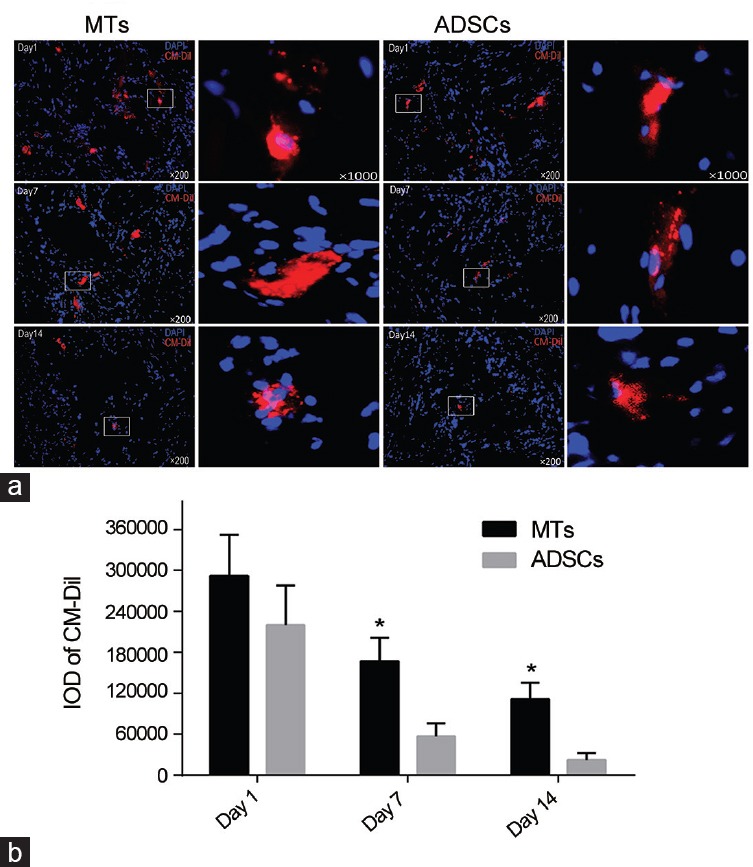
Time-dependent disappearance of IC-injected cells. (a) Representative images of CM-Dil-labeled cells retained in corpus cavernosum at days 1, 7, and 14 postinjection in ADSC- and MT-injected rats. Original magnification: Left, ×200 and right, ×1000. (b) Quantitative data of labeled cells between two groups are expressed as integrated optical density (IOD) of CM-Dil-positive area in corpus cavernosum. *P < 0.05 compared with DM + ADSCs group.
Erectile function assessment
Diabetic rats in DM + PBS group showed significant decreases in both maximum ICP and ICP to MAP ratios compared to the normal rats (P < 0.05). ADSCs treatment partially ameliorated erectile dysfunction compared with PBS-treated diabetic rats (P < 0.05) (Figure 3a) while MT-injected rats displayed significant improvements compared with ADSC-injected rats (P < 0.05). There were no striking differences in MAP between groups (Figure 3a and 3b).
Figure 3.
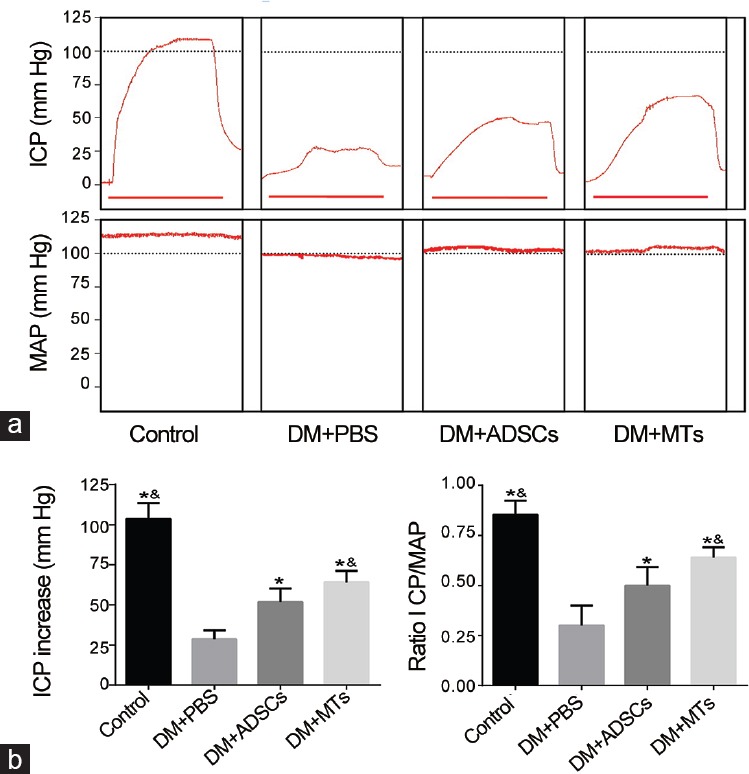
Erectile function of each group. (a) Representative images of intracavernous pressure (ICP) and mean arterial pressure (MAP). Horizontal bar below ICP represents 50 s of stimulate duration. (b) Left: Results of peak ICP increase of ADSCs- and MTs-treated rats. Right: Results of ratio ICP to mean MAP of each group. *P < 0.01 when compared with DM + PBS group. &P < 0.05 when compared with DM + ADSCs group.
Smooth muscle and endothelium contents in cavernous tissue
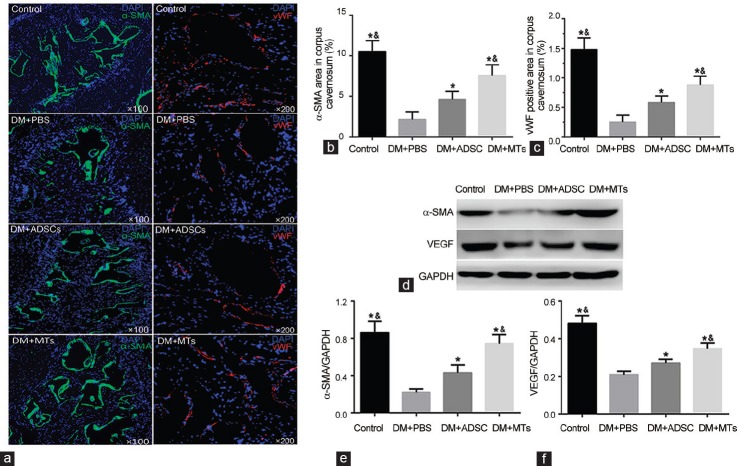
IF staining and WB analysis of α-SMA expression in penis showed a significant decrease of smooth muscle content in PBS-treated diabetic rats (Figure 4). Both ADSCs and MTs treatments restored the smooth muscle content (P < 0.05). Surprisingly, MTs treatment displayed a better recovery with the higher smooth muscle density (P < 0.05). The endothelium content was indicated by vWF-positive area in CC and markedly decreased in rats of DM + PBS group compared with rats in control group (P < 0.05). After IC injection of ADSCs, the endothelium content was increased to some degree (P < 0.05). After MTs treatment, the endothelium in cavernous tissue was better restored compared with ADSC-injected rats (P < 0.05) (Figure 4a and 4c). Meanwhile, WB results showed that VEGF expression in penis was higher in MT-injected rats than that of ADSC-injected rats (P < 0.05) or PBS-injected diabetic rats (P < 0.05) (Figure 4d and 4f).
Figure 4.
Smooth muscle and endothelium content. (a) Representative histological images of smooth muscle and endothelium in corpus cavernosum of each group indicated by α-SMA and vWF, respectively. Original magnification: ×100, ×200. Quantitative data of smooth muscle (b) and endothelium (c) are shown in right column bar graph. (d) Protein levels of α-SMA and VEGF are quantified as α-SMA/GAPDH (e) and VEGF/GAPDH (f) and expressed as mean ± standard deviation. *P < 0.05 compared with DM + PBS group. &P < 0.05 compared with DM + ADSCs group.
nNOS expressions in dorsal penile nerves and major pelvic ganglia
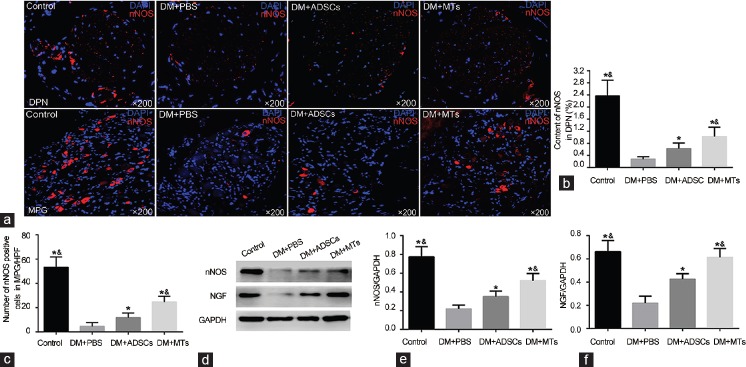
IF and WB results showed that the induced DM caused significant decreases of nNOS content in dorsal penile nerves (DPN) (P < 0.05) and nNOS-positive neurons in MPG (P < 0.05). Rats in DM + ADSCs group showed a partial recovery of nNOS expression (P < 0.05). Obviously, MTs injection restored more nNOS-positive nerves and neurons compared with ADSCs injection (P < 0.05) (Figure 5). Meanwhile, NGF expression in penis was higher in DM + MTs group than that of DM + ADSCs group or DM + PBS group (P < 0.05) (Figure 5d and 5f).
Figure 5.
nNOS expression in dorsal penile nerve and major pelvic ganglia. (a) Representative images of nNOS expression in the dorsal penile nerve (DPN) and nNOS-positive neurons in major pelvic ganglia (MPG), original magnification: ×200. Graph summarizing the quantitative data of nNOS content in DPN (b) and nNOS-positive cells in MPG (c). (d) Western blot analysis of nNOS and NGF contents in penis. Protein levels are quantified by nNOS/GAPDH (e) and NGF/GAPDH (f) in each column and expressed as mean ± standard deviation. *P < 0.05 compared with DM + PBS group. &P < 0.05 compared with DM + ADSCs group.
Rat cytokine array analysis of ADSCs and MTs
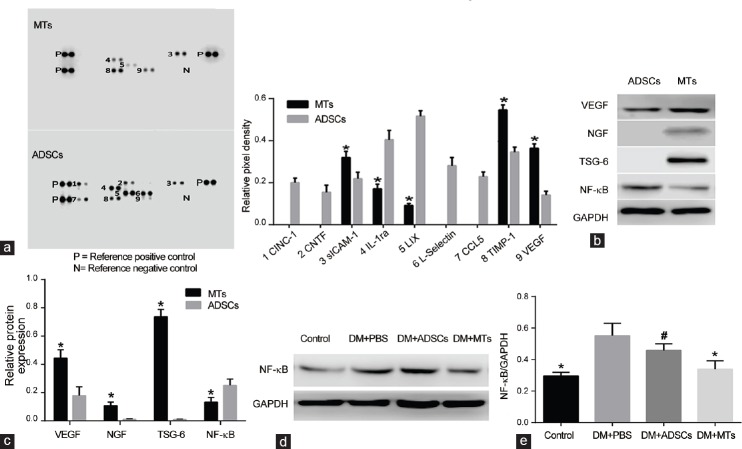
Rat cytokine array showed that cytokine-induced neutrophil chemoattractant 1 (CINC-1), cholinergic neurotrophic factor (CNTF), L-Selectin, and chemokine CCL5 were expressed only in ADSCs lysate, lipopolysaccharide-inducible CXC chemokine (LIX) and interleukin-1ra (IL-1ra) expressions were higher in ADSCs lysate than those of MTs lysate (P < 0.05), TIMP metallopeptidase inhibitor 1 (TIMP-1), and soluble intercellular cell adhesion molecule-1 (sICAM-1), and VEGF expressions were higher in MTs lysate (P < 0.05) (Figure 6a). WB results also demonstrated that VEGF expression was higher in MTs compared with ADSCs (P < 0.05), NGF and TSG-6 were only expressed in MTs, and NF-κB expression was lower in MTs (P < 0.05) (Figure 6b and 6c). Meanwhile, NF-κB expression in penis was higher in PBS-treated diabetic rats compared with rats in control group (P < 0.05) and ADSCs treatment did not affect the level of NF-κB (P > 0.05) while MTs treatment markedly decreased NF-κB expression (P < 0.05) (Figure 6d and 6e).
Figure 6.
Rat cytokine array analysis of ADSCs and MTs lysates. (a) Rat cytokine array analysis of MTs and ADSCs lysates showed that nine proteins were detected in ADSCs lysate while five proteins were detected in MTs lysate. Protein levels are showed as relative pixel density and quantified by positive control. *P < 0.05 compared with ADSCs lysate. (b) Western blot analysis of VEGF, NGF, TSG-6, and NF-κB contents in ADSCs and MTs. (c) Protein levels are quantified by VEGF/GAPDH, NGF/GAPDH, TSG-6/GAPDH, and NF-κB/GAPDH. *P < 0.05 compared with ADSCs. (d) Western blot analysis of NF-κB expression in penis of each group and (e) protein levels are quantified by NF-κB/GAPDH. *P < 0.05 compared with DM + PBS or DM + ADSCs group. #P > 0.05 compared with DM + PBS group.
DISCUSSION
Nitrergic nerves, endothelium, and smooth muscles are crucial for normal penile erection. The released nitric oxide (NO) from endothelium or nerve terminals stimulates the production of cyclic guanosine monophosphate (cGMP), and then the gradually accumulated cGMP helps dilate corporal smooth muscle, facilitate blood supply, and cause erection.23 Macrovasculopathy, microvasculopathy, and endothelial dysfunction are the major vasculopathy limiting the blood flow in the penis. Microvascular disease in diabetes also causes nerve ischemia resulting in peripheral neuropathy and autonomic neuropathy which play important roles in DMED. In the present study, reductions of smooth muscle and endothelium density in CC were observed in PBS-injected diabetic rats. Moreover, diabetic rats showed neuronal degeneration through markedly reduced nNOS content in DPN and nNOS-positive neurons in MPG. These results are consistent with previous findings about diabetic rats.10,24
ADSCs are adult stem cells with multipotency and low immunogenicity.22 Moreover, they have been applied to repair muscle tissues and restore tissue vascularization.25 As for ED therapy, independent groups have investigated the feasibilities and advantages of ADSCs in rat models of DMED, cavernous nerve injury, and Peyronie's disease.16,26,27 These results were encouraging but the traditional single-cell-based injection was relative preliminary, and the therapeutic potential was hampered by poor retention rate due to the blood flow. In this regard, MTs generated by a hanging drop method was seen as superior to single cell strategy. To examine whether IC injection of MTs has a better retention in diabetic rats, we labeled ADSCs with CM-Dil before treatment. The results showed that the labeled cells disappeared rapidly in CC of ADSC-injected rats as very limited amount was detected 2 weeks after IC injection while more CM-Dil-positive cells could be observed in MT-injected rats. These are consistent with the previous studies that rare cells could be tracked in cavernous tissue after transplantation and stem cells preprocessed as MTs could promote the cell retention and engraftment.15,19 The higher local retention could change the extracellular compartment of local tissue and might contribute to the greater improvement of erectile function.
Besides, many works have proposed that the transplanted cells played a part in CC might not through direct differentiation into local cell types but through the secretion of cytokines and growth factors.14,16,28 In this study, WB results demonstrated that the expressions of VEGF and NGF were higher in MTs than those of ADSCs. As a proangiogenic factor, VEGF has multiple functions including stimulates proliferation, inhibits apoptosis, and promotes cell survival.11 Impaired VEGF signaling pathway was closely related to endothelial dysfunction and was thought to be a possible cause for ED in diabetes.2 NGF is a type of neurotrophins that promote neural survival and differentiation. It has been revealed that NGF expression in target organs was decreased and the axonal transport of NGF was delayed in diabetic rats.29 VEGF and NGF expressions in penis were better augmented in MT-injected rats than those of ADSC-injected rats. Smooth muscle and endothelium density accompanied with nNOS content were increased more in MTs-treated rats than those of ADSCs-treated rats. These results demonstrated that MTs injection had better effects on vascularization and neuroprotection.
ADSCs expressed higher levels of the inflammatory proteins CINC-1, CCL5, and IL-1ra30 than MTs, indicating that MTs have anti-inflammatory activity. Indeed, MTs, but not ADSCs, expressed TSG-6, a multifunctional protein with an anti-inflammatory effect.31 Importantly, TSG-6 induced anti-inflammatory activity through the inhibition of transcript factor NF-κB, which regulated many key genes related to immune and inflammatory responses.32 WB results also showed the lower NF-κB expression in MTs compared with ADSCs, all of these indicated the anti-inflammatory ability of MTs. To examine whether IC injection of ADSCs or MTs inhibits local inflammation, we detected NF-κB expression in penis of each group. Interestingly, WB results demonstrated that diabetes-induced inflammation in penis and ADSCs treatment did not affect the local inflammation while the inflammatory state in CC was partially recovered by IC injection of MTs. This could be another reason for the higher efficiency of MTs treatment. Further studies should be carried out to check the systemic inflammation by measuring both pro- and anti-inflammatory cytokines to better understand the anti-inflammatory function of MTs.
Culturing adult stem cells in adherent monolayer could not truly recreate the specialized microenvironment which was known as stem cell niche in vivo and might limit the potential of transplanted cells.33 Therefore, mimicking the niche composition might help enhance the regenerative potential of ADSCs expanding in vitro.34 Moreover, subculturing of stem cells upon long-term in vitro would lead to phenotypes change and some abilities lose.19,35 In this study, both used single ADSCs and ADSCs dissociated from MTs possessed the adipogenic and osteogenic differentiation potential indicating that ADSCs retained their properties when cultured as MTs. Similarly, previous work on mesenchymal stem cells demonstrated that cells in spheroids retained most of the surface epitopes and differentiation potential.18 Nutrient deprivation and “microgravity” are the possible stimulating factors related to the changes of cells cultured as MTs. It is possible that stem cells cultured in MTs can contact closely with each other and the large amount of the cells ensures the quality of cell signaling and secretion. This can be partly supported by the higher expression of ICAM-1 in MTs since ICAM-1 was important in stabilizing cell-cell interactions.36
IC injection of ADSCs-based MTs resulted in a better improvement of erectile function in diabetic rats than single-cell ADSCs injection, and the greater therapeutic effects through enhanced paracrine abilities were supported by indirect evidence in our study. Moreover, the erectile function and histologic changes associated were not completely recovered only by ADSCs or MTs treatment. The diabetes status is relative complex and the hyperglycemia may cause sustained damages; therefore, blood glucose controlled by insulin combined with MTs injection will be a more promising way for DMED treatment. Investigations should be carried out in more ED animal models and to better evaluate the therapeutic effects and safety for potential clinical application.
AUTHOR CONTRIBUTIONS
JQH, ZCX, FZ, and YH conceived and designed the experiments. FZ, YH, YDX, HEL, RLG, and HX performed the experiments. BCY and ML involved in the retrieval and analysis of the data. YH, FZ, JQH, and ZCX participated in the drafting and final editing. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declared no competing interests.
ACKNOWLEDGMENTS
This study was funded by the National Natural Science Foundation of China (No. 81300478, 81270693), the Natural Science Foundation of Jiangsu Province (No. BK20130269), the Medical Science Foundation of Jiangsu Province Department of Health (No. H201312), the Science and Technology Development Program of Suzhou (No. SYS201450), China Postdoctoral Science Foundation (No. 2015M580466, 2016T90497).
REFERENCES
- 1.Kamenov ZA. A comprehensive review of erectile dysfunction in men with diabetes. Exp Clin Endocrinol Diabetes. 2015;123:141–58. doi: 10.1055/s-0034-1394383. [DOI] [PubMed] [Google Scholar]
- 2.Malavige LS, Levy JC. Erectile dysfunction in diabetes mellitus. J Sex Med. 2009;6:1232–47. doi: 10.1111/j.1743-6109.2008.01168.x. [DOI] [PubMed] [Google Scholar]
- 3.Hatzimouratidis K, Hatzichristou D. How to treat erectile dysfunction in men with diabetes: from pathophysiology to treatment. Curr Diab Rep. 2014;14:545. doi: 10.1007/s11892-014-0545-6. [DOI] [PubMed] [Google Scholar]
- 4.Thorve VS, Kshirsagar AD, Vyawahare NS, Joshi VS, Ingale KG, et al. Diabetes-induced erectile dysfunction: epidemiology, pathophysiology and management. J Diabetes Complications. 2011;25:129–36. doi: 10.1016/j.jdiacomp.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Long T, Liu G, Wang Y, Chen Y, Zhang Y, et al. TNF-alpha, erectile dysfunction, and NADPH oxidase-mediated ROS generation in corpus cavernosum in high-fat diet/streptozotocin-induced diabetic rats. J Sex Med. 2012;9:1801–14. doi: 10.1111/j.1743-6109.2012.02739.x. [DOI] [PubMed] [Google Scholar]
- 6.Ning H, Qiu X, Baine L, Lin G, Lue TF, et al. Effects of high glucose on human cavernous endothelial cells. Urology. 2012;80:1162.e7–11. doi: 10.1016/j.urology.2012.04.071. [DOI] [PubMed] [Google Scholar]
- 7.Wang L, Tian W, Uwais Z, Li G, Li H, et al. AGE-breaker ALT-711 plus insulin could restore erectile function in streptozocin-induced type 1 diabetic rats. J Sex Med. 2014;11:1452–62. doi: 10.1111/jsm.12533. [DOI] [PubMed] [Google Scholar]
- 8.Qiu XF, Li XX, Chen Y, Lin HC, Yu W, et al. Mobilisation of endothelial progenitor cells: one of the possible mechanisms involved in the chronic administration of melatonin preventing erectile dysfunction in diabetic rats. Asian J Androl. 2012;14:481–6. doi: 10.1038/aja.2011.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J, Zhou F, Li GY, Wang L, Li HX, et al. Evaluation of the effect of different doses of low energy shock wave therapy on the erectile function of streptozotocin (STZ)-induced diabetic rats. Int J Mol Sci. 2013;14:10661–73. doi: 10.3390/ijms140510661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun C, Lin H, Yu W, Li X, Chen Y, et al. Neurotrophic effect of bone marrow mesenchymal stem cells for erectile dysfunction in diabetic rats. Int J Androl. 2012;35:601–7. doi: 10.1111/j.1365-2605.2012.01250.x. [DOI] [PubMed] [Google Scholar]
- 11.Qiu X, Sun C, Yu W, Lin H, Sun Z, et al. Combined strategy of mesenchymal stem cell injection with vascular endothelial growth factor gene therapy for the treatment of diabetes-associated erectile dysfunction. J Androl. 2012;33:37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 12.Lotfy A, Salama M, Zahran F, Jones E, Badawy A, et al. Characterization of mesenchymal stem cells derived from rat bone marrow and adipose tissue: a comparative study. Int J Stem Cells. 2014;7:135–42. doi: 10.15283/ijsc.2014.7.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren ML, Peng W, Yang ZL, Sun XJ, Zhang SC, et al. Allogeneic adipose-derived stem cells with low immunogenicity constructing tissue-engineered bone for repairing bone defects in pigs. Cell Transplant. 2012;21:2711–21. doi: 10.3727/096368912X654966. [DOI] [PubMed] [Google Scholar]
- 14.Albersen M, Fandel TM, Lin G, Wang G, Banie L, et al. Injections of adipose tissue-derived stem cells and stem cell lysate improve recovery of erectile function in a rat model of cavernous nerve injury. J Sex Med. 2010;7:3331–40. doi: 10.1111/j.1743-6109.2010.01875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu X, Villalta J, Ferretti L, Fandel TM, Albersen M, et al. Effects of intravenous injection of adipose-derived stem cells in a rat model of radiation therapy-induced erectile dysfunction. J Sex Med. 2012;9:1834–41. doi: 10.1111/j.1743-6109.2012.02753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G, Sun X, Bian J, Wu R, Guan X, et al. Correction of diabetic erectile dysfunction with adipose derived stem cells modified with the vascular endothelial growth factor gene in a rodent diabetic model. PLoS One. 2013;8:e72790. doi: 10.1371/journal.pone.0072790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen XD, Dusevich V, Feng JQ, Manolagas SC, Jilka RL. Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J Bone Miner Res. 2007;22:1943–56. doi: 10.1359/jbmr.070725. [DOI] [PubMed] [Google Scholar]
- 18.Bartosh TJ, Ylostalo JH, Mohammadipoor A, Bazhanov N, Coble K, et al. Aggregation of human mesenchymal stromal cells (MSCs) into 3D spheroids enhances their antiinflammatory properties. Proc Natl Acad Sci U S A. 2010;107:13724–9. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frith JE, Thomson B, Genever PG. Dynamic three-dimensional culture methods enhance mesenchymal stem cell properties and increase therapeutic potential. Tissue Eng Part C Methods. 2010;16:735–49. doi: 10.1089/ten.TEC.2009.0432. [DOI] [PubMed] [Google Scholar]
- 20.Xu Y, Guan R, Lei H, Li H, Wang L, et al. Therapeutic potential of adipose-derived stem cells-based micro-tissues in a rat model of postprostatectomy erectile dysfunction. J Sex Med. 2014;11:2439–48. doi: 10.1111/jsm.12636. [DOI] [PubMed] [Google Scholar]
- 21.Huang YC, Shindel AW, Ning H, Lin G, Harraz AM, et al. Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol. 2010;183:1232–40. doi: 10.1016/j.juro.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. Adipose-derived stem cells: isolation, expansion and differentiation. Methods. 2008;45:115–20. doi: 10.1016/j.ymeth.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mas M. Molecular mechanisms of penile erection. Arch Esp Urol. 2010;63:589–98. [PubMed] [Google Scholar]
- 24.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–62. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 25.Alexeev V, Arita M, Donahue A, Bonaldo P, Chu ML, et al. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res Ther. 2014;5:21. doi: 10.1186/scrt411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Castiglione F, Hedlund P, Van der Aa F, Bivalacqua TJ, Rigatti P, et al. Intratunical injection of human adipose tissue-derived stem cells prevents fibrosis and is associated with improved erectile function in a rat model of Peyronie's disease. Eur Urol. 2013;63:551–60. doi: 10.1016/j.eururo.2012.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piao S, Kim IG, Lee JY, Hong SH, Kim SW, et al. Therapeutic effect of adipose-derived stem cells and BDNF-immobilized PLGA membrane in a rat model of cavernous nerve injury. J Sex Med. 2012;9:1968–79. doi: 10.1111/j.1743-6109.2012.02760.x. [DOI] [PubMed] [Google Scholar]
- 28.Garcia MM, Fandel TM, Lin G, Shindel AW, Banie L, et al. Treatment of erectile dysfunction in the obese type 2 diabetic ZDF rat with adipose tissue-derived stem cells. J Sex Med. 2010;7:89–98. doi: 10.1111/j.1743-6109.2009.01541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jakobsen J, Brimijoin S, Skau K, Sidenius P, Wells D. Retrograde axonal transport of transmitter enzymes, fucose-labeled protein, and nerve growth factor in streptozotocin-diabetic rats. Diabetes. 1981;30:797–803. doi: 10.2337/diab.30.10.797. [DOI] [PubMed] [Google Scholar]
- 30.Barsante MM, Cunha TM, Allegretti M, Cattani F, Policani F, et al. Blockade of the chemokine receptor CXCR2 ameliorates adjuvant-induced arthritis in rats. Br J Pharmacol. 2008;153:992–1002. doi: 10.1038/sj.bjp.0707462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartosh TJ, Ylostalo JH. Preparation of anti-inflammatory mesenchymal stem/precursor cells (MSCs) through sphere formation using hanging-drop culture technique. Curr Protoc Stem Cell Biol. 2014;28:Unit 2B 6. doi: 10.1002/9780470151808.sc02b06s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qiu JL, Zhu WL, Lu YJ, Bai ZF, Liu ZG, et al. The selective mGluR5 agonist CHPG attenuates SO2-induced oxidative stress and inflammation through TSG-6/NF-kappaB pathway in BV2 microglial cells. Neurochem Int. 2015;85-86:46–52. doi: 10.1016/j.neuint.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Saleh FA, Frith JE, Lee JA, Genever PG. Three-dimensional in vitro culture techniques for mesenchymal stem cells. Methods Mol Biol. 2012;916:31–45. doi: 10.1007/978-1-61779-980-8_4. [DOI] [PubMed] [Google Scholar]
- 34.Kim WS, Han J, Hwang SJ, Sung JH. An update on niche composition, signaling and functional regulation of the adipose-derived stem cells. Expert Opin Biol Ther. 2014;14:1091–102. doi: 10.1517/14712598.2014.907785. [DOI] [PubMed] [Google Scholar]
- 35.Ouyang A, Ng R, Yang ST. Long-term culturing of undifferentiated embryonic stem cells in conditioned media and three-dimensional fibrous matrices without extracellular matrix coating. Stem Cells. 2007;25:447–54. doi: 10.1634/stemcells.2006-0322. [DOI] [PubMed] [Google Scholar]
- 36.Schaefer A, Te Riet J, Ritz K, Hoogenboezem M, Anthony EC, et al. Actin-binding proteins differentially regulate endothelial cell stiffness, ICAM-1 function and neutrophil transmigration. J Cell Sci. 2014;127:4470–82. doi: 10.1242/jcs.154708. [DOI] [PubMed] [Google Scholar]