Figure 2.
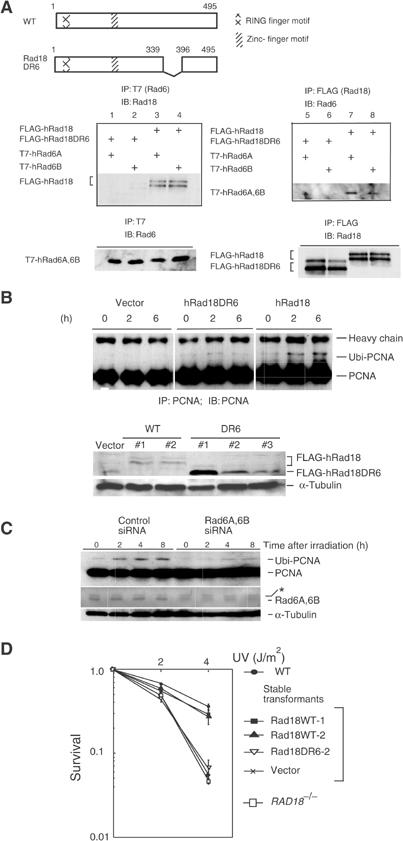
Requirement of Rad6A/B for monoubiquitination of PCNA in UV-irradiated cells. (A) Interaction of WT and mutant hRad18 with hRad6A/B. Full-length and mutant Rad18 proteins are schematically shown on the top panel. Plasmids were transfected into COS-7 cells with different combinations indicated on the left of the middle panels, and immunoprecipitation was performed. Similar levels of expression of hRad18 and hRad6A/B proteins in the transformed cells were confirmed in the lower panel. (B) Restoration of PCNA monoubiquitination in RAD18−/− cells by expression of WT hRad18 but not of mutant hRad18. Cells were incubated for 6 h following UV irradiation at 20 J/m2. Cell lysates were immunoprecipitated and blotted with an anti-PCNA antibody (upper panel). Expression of FLAG-hRad18 or FLAG-Rad18DR6 was confirmed in individual clones of stable transformants of RAD18−/− mouse fibroblasts by Western blot with an anti-Rad18 rabbit antibody (lower panel). α-Tubulin was indicated as a volume control. (C) Inhibition of PCNA monoubiquitination by siRNA for Rad6A/B. WI38VA13 cells were transfected with Rad6A and Rad6B siRNA, incubated for 4 days, and then irradiated with 10 J/m2 of UV light. At the indicated times, protein levels of monoubiquitinated PCNA were determined by Western blot. An asterisk shows a nonspecific band that remained constant following the siRNA treatment. (D) Restoration of UV sensitivity of RAD18−/− mouse cells by introduction of human Rad18 as determined by a colony-forming assay. Two independent clones of stable transformants (WT#1 and WT#2 in (B)) were tested.
