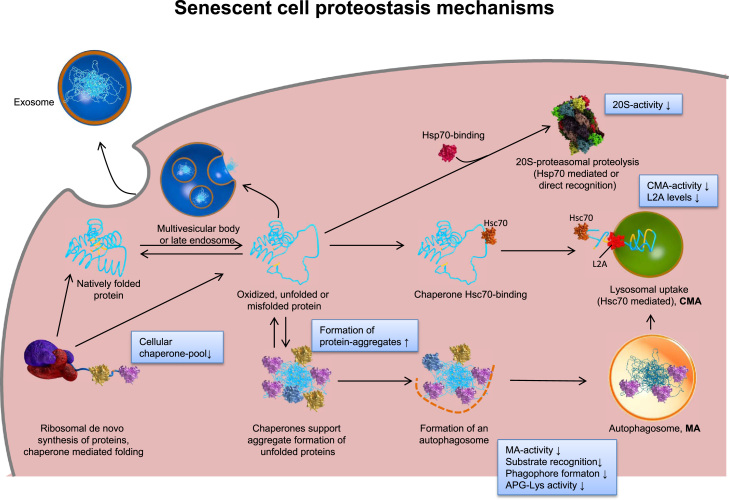
Fig. 4.
Proteostasis changes in a senescent cell. The scheme shows the overall changes of the cellular systems maintaining protein homeostasis (proteostasis) and thus, cellular functionality during aging. The main proteolytic systems, responsible for recognition and degradation of un/misfolded or oxidatively damaged proteins are the proteasomal system, involving the ATP-dependent 26S proteasome as well as the ATP-independent 20S proteasome, and autophagy (including both autophagy (MA) as well as the chaperone mediated one (CMA)). Damaged proteins can be directly recognized as substrates by the 20S proteasome, resulting in proteolytic removal from the cell. Furthermore, they can be recognized by chaperones or heat shock proteins that keep their substrates in a soluble state, preventing the formation of aggregates. Another fate might be the formation of aggregates, driven by hydrophobic residues exposure. Such aggregates can be incorporated into an autophagosome and fusing with lysosomes, resulting in proteolytic degradation by lysosomal proteases, or they can be removed from the cell via excretion as exosome. Modified from [115] and according to [122].