Abstract
Remodelling in the atria could appear as a result of hypertension, diabetes or ischaemic heart disease. Galectin-3 (Gal-3) is a mediator of profibrotic pathways and a potential biomarker of cardiac remodelling. We prospectively recruited consecutive patients undergoing elective cardiac surgery. Preoperative Gal-3 levels were determined from serum samples, and the presence of fibrosis was assessed from atrial appendage tissue samples obtained during cardiac surgery. We included 100 patients with aortic valve or ischaemic heart diseases and 15 controls with permanent AF. Gal-3 levels were associated with sex, left atrial volume, previous cardiac disease, diabetes mellitus, hypertension, NYHA and NT-proBNP. We observed differences in serum Gal-3 concentrations between patients and controls with permanent AF (p = 0.020). We performed ROC curves related to fibrosis and established a cutoff point for Gal-3 >13.65 ng/ml. Multivariate analyses showed previous cardiac disease, NYHA scale and high Gal-3 to be independent predictors of fibrosis. After adjustment for confounding factors, atrial fibrosis remained the only independent factor for the development of AF (p = 0.022). High Gal-3 serum levels predict fibrosis of the atrial appendage. NYHA scale and previous cardiac disease were also associated with tissue fibrosis in patients undergoing surgery. Atrial fibrosis was the only independent predictor for post-operative AF occurrence in our model after correcting for confounding factors.
Changes in atrial function and structure are known as atrial remodelling, which contributes to the development of atrial fibrillation (AF)1. AF is associated with an increased morbidity and mortality after cardiac surgery and a longer hospital length of stay2,3. A significant association between fibrosis, inflammation, oxidative stress and the development, recurrence and perpetuation of AF has been hypothesized4.
AF after cardiac surgery occurs in approximately 20–50% of patients undergoing cardiac surgery5. Numerous predisposing factors such as advanced age, hypertension, diabetes, left atrial enlargement, left ventricular hypertrophy, or intra-operative and post-operative factors such as atrial injury or ischaemia, have been associated with the development of post-operative AF6.
Galectin-3 (Gal-3) is a beta-galactoside binding lectin that appears to be a mediator of cardiac fibrosis in a number of recent experimental studies. Gal-3 has been reported as a mediator of profibrotic pathways and as a potential biomarker of adverse cardiac remodelling7. Gal-3 is expressed by activated macrophages and induces cardiac fibroblasts to proliferate and deposit type I collagen in the myocardium8,9. This protein is linked to areas of fibrosis, suggesting an active role in modulation of the extracellular matrix. Therefore, Gal-3 appears to link pathways of inflammation and fibrosis, and it may contribute to the development of heart failure. Its role in atrial remodelling has scarcely been studied, and it was the main purpose for the present manuscript.
Results
We included 100 patients with predominantly aortic valve (n = 42) or ischaemic heart (n = 58) diseases and 15 controls with permanent AF, all of whom underwent cardiac surgery. Twenty-nine patients (29%) developed post-surgical AF, of whom 13 occurred during the Intensive Care Unit stay. Aortic patients showed a higher rate of AF development than coronary patients (41.5% vs 20.7%, p = 0.026). Patients developing AF had a longer stay in the Intensive Care Unit (p = 0.008) as well as longer overall hospitalization stays (p = 0.005).
We observed differences in serum Gal-3 concentrations between patients and controls with permanent AF (14.25 ± 4.15 vs 17.61 ± 6.84 ng/mL; p = 0.020) (Table 1). The maximum and minimum Gal-3 levels were 23.10–7.00 for patients and 24.30–8.00 for controls. No differences between aortic and coronary patients (14.71 ± 4.34 vs 13.91 ± 4.02 ng/mL; p = 0.402) were observed. In a univariate regression model, Gal-3 was significantly associated with age, sex, left atrial volume, previous cardiac disease, diabetes mellitus, hypertension, and NYHA functional class. In multivariate analysis, only sex, previous cardiac disease and diabetes mellitus remained independent predictors for Gal-3 values (all p < 0.05; Table 2). We evaluated correlations with NT-proBNP values, an established biomarker of wall stress and cardiac remodelling, and we found a significant positive correlation: r = 0.226, p = 0.045.
Table 1. Baseline characteristics of included patients (n: 100).
| Clinical or demographic variable | %, mean ± sd or median (IQR) |
|---|---|
| Age (years) | 65.1 ± 9.5 |
| Male | 77 |
| Coronary patient | 58 |
| Aortic Valvular patient | 42 |
| Smoking habit | 23 |
| Hypertension | 70 |
| Hypercholesterolemia | 65 |
| Diabetes mellitus | 47 |
| DM insulin-dependent | 12 |
| DM under oral treatment | 38 |
| Previous pulmonary chronic obstructive disease | 8 |
| Previous thyroid disease | 6 |
| Previous stroke | 9 |
| NYHA | 2 (1–2) |
| EuroSCORE | 4 (2–5) |
| Left atrial diameter (mm) | 40.71 ± 5.80 |
| Left atrial volume (Ellipsoidal; mm3) | 27.08 ± 8.81 |
| Aortic clamp time (min) | 46.3 ± 16.8 |
| Cardiopulmonary bypass pump time (min) | 90.37 ± 28.76 |
| AF development in the Intensive Care Unit | 13 |
| Total hospitalization time (days) | 9 (8–16) |
| Stay in the Intensive Care Unit (days) | 2 (2–4) |
| Stay in the Cardiology Department (days) | 7 (5–14) |
| Galectin concentration (mg/mL) | 14.25 ± 4.15 |
EuroSCORE: The European System for Cardiac Operative Risk Evaluation. NYHA: New York Heart association functional class.
Table 2. Linear regression (stepwise mode) analysis for the predictors of Galectin-3 values.
| Univariate analysis B-coefficient (95% CI); p value | Multivariate analysis B-coefficient (95% CI); p value | |
|---|---|---|
| Age | 0.11 (0.02–0.21); 0.023 | 0.140 |
| Gender, male | 2.75 (0.65–4.85); 0.011 | 2.88 (0.81–4.95); 0.007 |
| Left atrial volume | 0.10 (−0.10–0.21); 0.073 | 0.127 |
| Previous cardiac disease | −2.56 (−4.67 to −0.45); 0.018 | −2.19 (−4.31 to −0.07); 0.044 |
| Diabetes mellitus | 2.37 (0.58–4.17); 0.010 | 2.08 (0.29–3.87); 0.024 |
| Hypertension | 2.66 (0.69–4.63); 0.009 | 0.050 |
| NYHA scale | 1.01 (0.−0.35–2.37); 0.144 | 0.151 |
| Smoking habit | −0.013 (−2.20–2.18); 0.991 | |
| CrCl | −1.06 (−5.84–3.72); 0.660 | |
| Dyslipidaemia | 0.69 (−1.30–2.67); 0.494 |
NYHA: New York Heart association functional class CrCl: Creatinine clearance.
Gal-3 levels as a predictor for atrial fibrosis
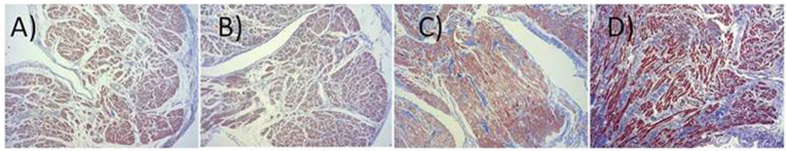
When evaluating atrial appendage interstitial fibrosis using Masson’s trichrome as indicated, 5 (4.5%) tissue samples showed low fibrosis (connective tissue infiltration Grade 1), 37 (40.2%) showed medium fibrosis (connective tissue infiltration Grade 2), and 50 (54.3%) showed intensive interstitial fibrosis (connective tissue infiltration Grade 3) (Fig. 1A–D). Eight samples were not evaluable due to different technical reasons. We performed ROC curves related to high-grade fibrosis (AUC: 0.630 ± 0.069 (CI95%: 0.494–0.762); p: 0.06; Fig. 2) and established a cutoff point for Gal-3 >13.65 ng/ml. Univariate analysis showed that age, previous cardiac disease, septal thickness, NYHA scale, creatinine clearance (CrCl) and high Gal-3 were associated with fibrosis (Table 3). Likewise, in multivariate analyses, we observed that previous cardiac disease, NYHA scale and high Gal-3 [OR (95%CI): 4.37 (1.16–16.41), p = 0.029; 2.93 (1.26–6.85), p = 0.013 and 3.29 (1.07–10.11), p = 0.037, respectively] remained independent predictors of fibrosis (Table 3).
Figure 1.
Classification of the myocardial fibrosis assessed by Masson’s trichrome staining infiltration grades: grade 0 (A), 1 (B), 2 (C) and 3 (D).
Figure 2. Receiver Operating Characteristic (ROC) for evaluation of GAL-3 levels related to AF development in patients undergoing cardiac surgery.
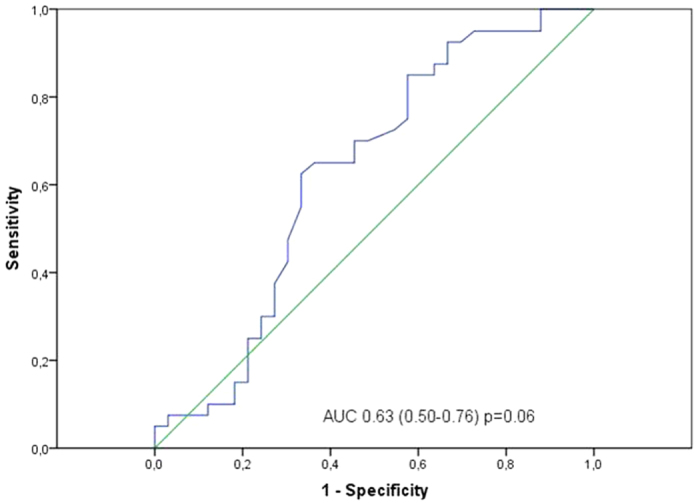
Table 3. Logistic regression analysis for fibrosis presence.
| Univariate analysis OR (95%CI); p value | Multivariate analysis OR (95%CI); p value | |
|---|---|---|
| Age | 1.03 (0.99–1.08); p = 0.105 | 1.21 (1.15–3.08); p = 0.523 |
| Gender, male | 1.15 (0.04–3.09); p = 0.770 | |
| Hypertension | 1.28 (0.52–3.13); p = 0.580 | |
| Diabetes mellitus | 1.12 (0.53–2.81); p = 0.622 | |
| Previous cardiac disease | 2.44 (0.96–6.19); p = 0.059 | 4.37 (1.16–16.41); p = 0.029 |
| Left atrial volume | 0.99 (0.95–1.04); p = 0.949 | |
| Septum thickness | 16.85 (2.01–136.36); p = 0.008 | 8.23 (0.30–233.0); p = 0.211 |
| Dyslipidaemia | 1.58 (0.66–3.76); p = 0.295 | |
| Smoking habit | 1.03 (0.38–2.79); p = 0.947 | |
| NYHA scale | 2.05 (1.09–3.83); p = 0.024 | 2.93 (1.26–6.85); p = 0.013 |
| CrCl | 0.98 (0.96–1.00); p = 0.062 | 0.99 (0.97–1.01); p = 0.689 |
| Galectin-3 >13.65 ng/mL | 3.33 (1.26–8.76); p = 0.015 | 3.29 (1.07–10.11); p = 0.037 |
NYHA: New York Heart association functional class CrCl: Creatinine clearance.
Interstitial fibrosis in the prediction of atrial fibrillation occurrence
We also proposed to evaluate the incidence of tissue remodelling in the occurrence of AF in patients after cardiac surgery. Demographic factors including age and sex, clinical variables such as body mass index, left atrial volume, EuroSCORE index or type of surgery, and interstitial atrial fibrosis were associated with AF occurrence in a logistic regression analysis [OR (95%CI): 1.09 (1.00–1.11), p = 0.048; 4.96 (1.84–13.36), p = 0.031; 1.09 (0.98–1.21), p = 0.123; 1.06 (1.00–1.11), p = 0.042, 1.26 (1.03–1.54), p = 0.025; 2.61 (1.08–6.32), p = 0.034 and 2.18 (0.88–5.44), p = 0.094, respectively]. After adjustment for potential confounding factors, only atrial remodelling evaluated as tissue atrial fibrosis remained an independent factor for AF development [OR (95%CI): 3.77 (1.20–11.76), p = 0.022; Table 4] in our multivariate model. When reanalyzing by excluding subsequent confounding factors to avoid a problem of “over-fitting,” only the atrial fibrosis remained as an independent factor (data not shown).
Table 4. Logistic regression for the prediction of AF occurrence.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (CI95%) | p | HR (CI95%) | p | |
| Age | 1.09 (1.00–1.11) | 0.048 | 1.00 (0.93–1.08) | 0.951 |
| Male sex | 4.96 (1.84–13.36) | 0.031 | 2.14 (0.58–7.88) | 0.252 |
| Hypertension | 1.51 (0.56–4.03) | 0.415 | ||
| Hypercholesterolemia | 2.05 (0.77–5.42) | 0.150 | ||
| Diabetes mellitus | 1.31 (0.55–3.10) | 0.546 | ||
| Body mass index | 1.09 (0.98–1.21) | 0.123 | 1.06 (0.92–1.22) | 0.443 |
| Indexed left atrial volume | 1.06 (100–1.11) | 0.042 | 1.03 (0.96–1.10) | 0.380 |
| Left ventricular ejection fraction | 0.99 (0.96–1.03) | 0.797 | ||
| Clamping time | 0.98 (0.96–1.01) | 0.235 | ||
| Cardiopulmonary pump time | 0.99 (0.98–1.01) | 0.679 | ||
| Type of surgery (valve surgery vs CABG) | 2.61(1.08–6.32) | 0.034 | 2.73(0.63–11.86) | 0.181 |
| EuroSCORE | 1.26 (1.03–1.54) | 0.025 | 1.28 (0.91–1.79) | 0.150 |
| Interstitial atrial appendage fibrosis | 2.18 (0.88–5.44) | 0.094 | 3.77 (1.20–11.76) | 0.022 |
CABG: coronary artery bypass grafting; EuroSCORE: The European System for Cardiac Operative Risk Evaluation; ACE: angiotensin-converting enzyme; ARBs: angiotensin receptor blockers; CA: calcium antagonists.
Discussion
Gal-3 has been reported to be a biomarker for cardiac fibrosis. Our results support previous studies since we found a clear association between Gal-3 serum levels and interstitial fibrosis measured in the right atrial appendage obtained by resection.
Fibrosis is a result of structural remodelling, and it has been proposed as the main arrhythmogenic substrate perpetuating AF10. In our cohort, 20% of patients showed clinical AF during their hospitalization stay after coronary surgery. This finding is consistent with previous data indicating AF occurrence in approximately 20–50% of patients undergoing cardiac surgery (5). Pre-surgical levels of Gal-3 suggest an active remodelling process in the atrial appendage tissue prior to the surgical intervention. In addition, we also found a positive correlation between Gal-3 and NT-proBNP levels as a biomarker of established wall stress and cardiac remodelling, supporting our hypothesis. In previous studies, we observed that pre-surgical hsTnT11 and vWF levels12 were indicators of ongoing subclinical myocyte damage, endothelial dysfunction and remodelling in the atria. Both biomarkers resulted were therefore associated with AF development in patients undergoing cardiac surgery. Here, we found an association between Gal-3 levels and fibrosis when analyzing all the available samples. Our results indicate that Gal-3 pro-fibrotic effects and interstitial atrial remodelling are converging processes in fibrosis development. Whether or not this fibrosis is the responsible cause for AF development, is an assumption that we cannot demonstrate with our results, but is supported by the clear association between fibrosis and AF development. Our data describing higher values of left atrial volume and septal thickness in both patients presenting with intensive fibrosis and in patients who developed AF also reinforce the same idea. In this context, surgery would act as a trigger for the development of AF in a predisposed environment involving highly remodelled tissue.
In addition, we found higher Gal-3 levels in a positive control cohort of permanent AF patients, supporting our hypothesis. Recent studies have found a correlation between Gal-3 levels and atrial remodelling, including the extent of left atrial fibrosis and atrial electromechanical properties13.
This study is limited by its observational design; we could explore only associations, and no causality is implied. The recruitment protocol did not guarantee the exclusion of patients with previously silent AF from the study. Although Gal-3 level has been proposed as a biomarker of fibrosis in cardiovascular diseases, we cannot ignore possible changes in Gal-3 levels over time. Another limitation is related to the studied tissue samples, as we had no access to left atrial appendage tissue.
In conclusion, we can summarize that Gal-3 levels are higher in controls with permanent AF versus patients without previous known AF undergoing cardiac surgery. High Gal-3 serum values predict fibrosis of the right atrial appendage. Other clinical factors such as NYHA scale and previous cardiac disease were also associated with the presence of fibrosis in patients undergoing surgery. Atrial fibrosis was the only independent predictor for post-operative AF occurrence in our model, even after correcting for confounding factors.
Methods
Patients
We prospectively recruited consecutive patients undergoing elective cardiac surgery with cardiopulmonary bypass from November 2010 until February 2012. We excluded patients with previous AF (paroxysmal or permanent), unstable angina, hepatic or renal failure (creatinine clearance <50 ml/min), and chronic inflammatory or neoplasic diseases. Patients undergoing urgent surgery and those with a previous history of pacemakers, infectious endocarditis and those undergoing AF-related surgery were also excluded.
We documented AF during the post-operative period in the Intensive Care Unit by continuous 3-derivations telemetry and by a Holter device once the patient was in the hospitalization cardiac surgery unit. Holter monitoring was extended until a maximum of 10 days after surgery. In addition, a 12 derivations electrocardiogram was performed in symptomatic patients and daily during the hospitalization. AF development was defined as an episode of AF lasting for more than 2 minutes in any of the ECG registries.
All echocardiographic measurements were performed off-line by the same accredited cardiologist who was unaware of clinical and laboratory data14. Left atrial volume was calculated according to the ellipsoid model that assumes that the left atrium can be adequately represented as a prolate ellipsoid with a volume of 4p⁄3(L⁄2)(D1⁄2)(D2⁄2), where L is the long-axis (ellipsoid) and D1 and D2 are the orthogonal short-axis dimensions15. Left atrial volume calculations were indexed to body surface area calculated according to Gehan and George16.
The study was carried out according to the principles of the Declaration of Helsinki and was approved by the Ethics Committee of the Hospital Universitario Virgen de la Arrixaca. All the included patients gave informed consent to participation.
Blood samples and laboratory assays
Venepuncture was performed the morning of cardiac surgery with the patient fasting for >12 hours. We collected samples immediately before cardiac surgery. Plasma fractions were obtained by centrifugation for 15 minutes at 3500 × g. Aliquots were stored at −40 °C to allow batch analysis in a blinded fashion.
Preoperative Gal-3 levels were determined in defrosted serum samples by ELFA (Enzyme-Linked Fluorescent Assay) in a MiniVidas analyzer (BioMérieux®, France). The inter-assay and intra-assay coefficients of variation were 6.5% and 1.6%, respectively. The measured range was 3.3–100 ng/mL, the lower limit of detection was 2.2 ng/mL and the limit of quantification was 3.3 ng/mL. Levels of preoperative NT-proBNP were measured as described elsewhere11.
Obtaining and staining right atrial appendage tissue
Atrial appendage tissue was obtained during surgery by cannulation for the extracorporeal circulation. This cannulation was performed directly into the right atria with non-absorbable suture in a “tobacco bag” shape. To provide adequate cannula apposition, the bag was opened and cut. The remaining appendage tissue was collected for the tissue study objectives.
All recruited subjects gave their informed consent to participate in the study. All surgical procedures were performed under cardiopulmonary bypass, with mild hypothermia (30 °C), cardioplegic arrest of the heart and left ventricular (LV) venting through the right superior pulmonary vein. We used anterograde and retrograde cold intermittent blood cardioplegia (Cardi-Braun®; B-Braun, Inc., Barcelona, Spain) for myocardial protection.
The tissue samples were processed, paraffin embedded and cut into 2–3 μm sections. For histochemical evaluation of connective tissue infiltration within the myocardial tissues, a Masson’s trichrome staining was performed on sections from affected specimens by an automatized staining system (Dako Artisan, Dako, Carpinteria, California, USA) following the manufacturer’s recommendations. The degree of connective tissue infiltration was measured using a qualitative scale from 0 to 3 (0 was negative; 1, mild; 2, medium; and 3, high infiltration) at the location within the tissue (perivascular or interstitial fibrosis). All assessments were blinded and performed twice to ensure the repeatability of the results. The analysis was made using an Axio Scope A1 transmitted-light microscope (Carl Zeiss, Jena, Germany).
Statistical analysis
Categorical variables are presented as counts (percentages), while continuous variables are presented as the mean ± SD (standard deviation) or median (25th–75th percentiles), as appropriate. The Kolmogorov-Smirnov test was used to check for normal distribution of continuous data.
Variables associated with Gal-3 values were studied by linear regression analysis (stepwise mode). We constructed areas under the receiver-operator characteristic (ROC) curve for Gal-3 related to high grade fibrosis. The cutoff point with the best sensitivity and specificity was chosen for each case, as assessed by ROC curves. Gal-3 levels were dichotomized as ‘low’ or ‘high’ according to whether the circulating levels were under or over the calculated threshold (cutoff). This dichotomy for Gal-3 levels was assessed via the logistic regression model to explore the overall association between fibrosis in tissues obtained by myectomy and Gal-3 values. We considered fibrosis to be intensive when the degree of connective tissue infiltration was 2 or 3 with Masson’s trichrome stain. Logistic regression analyses were performed to assess the predictive variables for AF development including fibrosis. All p values < 0.05 were accepted as statistically significant. Statistical analysis was performed using SPSS 19.0 for Windows (SPSS, Inc., Chicago, IL, USA).
Additional Information
How to cite this article: Hernández-Romero, D. et al. Galectin 3 as marker of interstitial atrial remodeling involved in atrial fibrillation. Sci. Rep. 7, 40378; doi: 10.1038/srep40378 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
We want to particularly acknowledge the patients and the BioBank “Biobanco en Red de la Región de Murcia” (PT13/0010/0018) integrated in the Spanish National Biobanks Network (B.000859) for its collaboration. This work was supported by the Instituto de Salud Carlos III and in part by Funding FEDER (RETIC, RD12/0042/0049; FIS, PI13/00513) and the Instituto Murciano de Investigaciones Biosanitarias Virgen de la Arrixaca (IMIB, Murcia, Spain) (grant numbers RAICMUR_012/2014 to AR, APOYO_041/2014 to EOP, CM11/00149 to JAV and RD12/0042/0049 to DHR).
Footnotes
Author Contributions D.H.R. designed the study, performed biological samples processing and analysis, wrote the manuscript and acted as the corresponding author. J.A.V. determined Gal-3 values and contributed to the scientific discussion. A.L. recruited patients and performed surgeries. A.G.A. monitored patients for A.F. occurrence. A.I.R.A. collected samples and clinical data, and E.J. contributed to tissue handling and processing. R.J.R. was responsible for patient follow-up during their stay at the intensive care unit. C.M.M. performed tissue evaluation for fibrosis, M.V. provided funding and collaborated in the discussion and F.M. designed the study, provided funding and coordinated the work. All authors reviewed the manuscript.
References
- Nattel S., Burstein B. & Dobrev D. Atrial remodelling and atrial fibrillation: mechanisms and implications. Circ Arrhythm Electrophysiol. 1, 62–73 (2008). [DOI] [PubMed] [Google Scholar]
- Go A. S. et al. Prevalence of diagnosed atrial fibrillation in adults. National implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) study. JAMA. 285, 2370–5 (2001). [DOI] [PubMed] [Google Scholar]
- Arribas-Leal J. M. et al. Epidemiology and new predictors of atrial fibrillation after coronary surgery. Rev Esp Cardiol. 60, 841–7 (2007). [PubMed] [Google Scholar]
- Benjamin E. J. et al. Independent risk factors for atrial fibrillation in a population-based cohort: the Framingham Heart Study. JAMA. 271, 840–4 (1994). [PubMed] [Google Scholar]
- Orenes-Piñero E. et al. Pre and post-operative treatments for prevention of atrial fibrillation after cardiac surgery. Mini Rev Med Chem. 12, 1419–31 (2012). [DOI] [PubMed] [Google Scholar]
- Sánchez-Quiñones J., Marín F., Roldán V. & Lip G. Y. The impact of statin use on atrial fibrillation. QJM. 10, 1845–61 (2008). [DOI] [PubMed] [Google Scholar]
- de Boer R. A., Yu L. & van Veldhuisen D. J. Galectin-3 in cardiac remodelling and heart failure. Curr Heart Fail Rep. 7, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma U. C. et al. Galectin-3 marks activated macrophages in failureprone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 110, 3121–3128 (2004). [DOI] [PubMed] [Google Scholar]
- Liu Y. H. et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodelling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 296, H404–H412 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allessie M., Ausma J. & Schotten U. Electrical, contractile and structural remodelling during atrial fibrillation. Cardiov Res. 54, 230–46 (2002). [DOI] [PubMed] [Google Scholar]
- Hernández-Romero D. et al. High-sensitivity troponin T as a biomarker for the development of atrial fibrillation after cardiac surgery. Eur J Cardiothorac Surg. 45, 733–8 (2014). [DOI] [PubMed] [Google Scholar]
- Hernández-Romero D. et al. VonWillebrand factor is associated with atrial fibrillation development in ischaemic patients after cardiac surgery. Europace. Nov 12, pii: euv354 [Epub ahead of print] (2015). [DOI] [PubMed] [Google Scholar]
- Yalcin M. U. et al. J Cardiovasc Electrophysiol. 26, 635–40 (2015). [DOI] [PubMed] [Google Scholar]
- Fox K. F. et al. Report on the European Association of Echocardiography accreditations in echocardiography: December 2003–September 2006. Eur J Echocardiogr. 8, 74–9 (2007). [DOI] [PubMed] [Google Scholar]
- Lang R. M. et al. Recommendations for chamber quantification. Eur J Echocardiogr. 7, 79–108 (2006). [DOI] [PubMed] [Google Scholar]
- Gehan E. A. & George S. L. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 54, 225–35 (1970). [PubMed] [Google Scholar]