Abstract
The aim of the present study was to evaluate the value of shear wave elastography (SWE) and contrast-enhanced ultrasonography (CEUS) in the diagnosis of thyroid malignant nodules. A total of 253 patients with 319 thyroid nodules were subjected to two-dimensional ultrasound (2DUS) and CEUS examinations prior to thyroidectomy between March, 2014 and December, 2015. Young's modulus value for each nodule on 2DUS and CEUS images were recorded. The sensitivity, specificity and accuracy of 2DUS, SWE and CEUS in the diagnosis of thyroid malignant nodules were assessed. The results demonstrated that, of the 319 nodules that were pathologically confirmed, 183 were malignant and 136 were benign. The area under the receiver operating characteristic curve as a result of SWE diagnosis was 0.77. When the threshold of the Young's modulus value was ≥27.65 kPa in the diagnosis of malignant thyroid nodules, SWE exhibited a sensitivity of 84.55% (115/136), a specificity of 84.15% (154/183) and an accuracy of 84.32% (269/319). US contrast imaging of malignant thyroid nodules revealed a major tendency for early hypoenhancement and hypoenhancement. CEUS exhibited a sensitivity of 87.5% (119/136), a specificity of 86.33% (158/183) and an accuracy of 86.83% (277/319) in the diagnosis of malignant thyroid nodules. Compared with 2DUS, SWE, CEUS and their combined use exhibited statistically significant differences in the diagnosis of thyroid malignant nodules in terms of sensitivity, specificity and accuracy (χ2=9.220,15.310 and 40.296, respectively; P=0.000); SWE or CEUS did not differ significantly in the diagnosis of thyroid malignant nodules in terms of sensitivity, specificity or accuracy (χ2=0.737;P=0.542); Compared with the use of SWE or CEUS alone, their combination exhibited statistically significant differences in the diagnosis of malignant thyroid nodules in terms of sensitivity, specificity and accuracy (χ2=12.264 and 6.939, respectively; P=0.000,0.005). In conclusion, the high accuracy of the combined use of SWE and CEUS in the diagnosis of malignant thyroid nodules is of great clinical value.
Keywords: ultrasonography, contrast media, thyroid nodule, malignant, benign
Introduction
Thyroid nodules have an incidence rate ranging between 19 and 67% among different populations. Thyroid nodules are the most commonly encountered tumors in the neck area, with malignant nodules accounting for ~5–10% (1–3). It has been reported that 1/3 of thyroid tumors are prone to lymph node metastasis (4); therefore, early diagnosis and timely treatment of thyroid nodules significantly affect recovery and outcome. High-frequency ultrasonography (US) is a priority imaging choice for identifying benign and malignant thyroid nodules (5). With the increased use of real-time shear wave elastography (SWE) and contrast-enhanced ultrasonography (CEUS) in the diagnosis of diseases of the liver, prostate, breast and other organs (6–8), their combined use also provides a new approach to the diagnosis of thyroid disease. The aim of the present study was to investigate the value of the combined use of real-time (SWE) and CEUS in the diagnosis of benign and malignant thyroid nodules.
Subjects and methods
Subjects
A retrospective analysis of 319 pathologically confirmed thyroid nodules in 253 patients who underwent surgery between March, 2014 and December, 2015 was conducted. All the patients were subjected to two-dimensional US (2DUS), SWE and CEUS imaging. A total of 86 male patients, aged 19–76 years [mean ± standard deviation (SD), 44±13 years] were found to carry a total of 116 nodules; 167 female patients, aged 18–78 years (mean ± SD, 43±11 years) were found to carry a total of 203 nodules. The mean ± SD of the maximum diameters of the 319 nodules was 11.3±2.2 mm (range, 3–67 mm), with a median of 12.5 mm.
The present study was conducted in accordance with the Declaration of Helsinki and with approval of the Ethics Committee of Ningbo First Hospital (Ningbo, China). Written informed consent was obtained from all the participants.
Instruments and methods
2DUS
The patients underwent SWE performed using a linear 4–15 MHz transducer (Aixplorer, SuperSonic Imagine, Les Jardins de la Duranne, France). 2DUS was used to determine thyroid nodule location, size, internal echo, calcification, borders and pattern, and to calculate the nodule diameter and longitudinal diameter ratio (aspect ratio). Subsequently, the SWE mode was added. The patients were asked to hold their breath when the probe was lightly applied until the image stabilized. Three continuous measurements of the lesion were then taken and averaged. The sampling frame should be 2–3 times greater than the area of the lesion, with Q-BOX covering the latter within a range of 0–180 kPa.
Ceus
The Philips iU22 Xmatrix ultrasonography diagnostic apparatus with an L9-3 probe was used (Koninklijke Philips N.V., Amsterdam, The Netherlands). The instrument was equipped with UE and CEUS analysis software. The patients were asked to lie in the supine position. Prior to US, two-dimensional images and color Doppler images of the thyroid nodules were collected. Subsequently, intravenous access was established through an elbow vein. A total of 25 mg SonoVue (Bracco Imaging S.p.A, Milan, Italy) was dissolved in 5 ml 0.9% sodium chloride and was injected as an intravenous bolus of 1.2 ml per subject via the antecubital vein, followed by 5 ml 0.9% sodium chloride. Under the US contrast mode the mechanical index (MI) ranged between 0.05 and 0.06, and the focal point was adjusted to the middle or the lower edge of the thyroid nodules. If the nodule is small, the double-contrast mode maybe used to ensure the probe is in parallel with the long axis of the thyroid gland as much as possible. During the entire process, the patients were asked to refrain from swallowing, and the probe was fixed to the largest section of the nodule in order to ensure a continuous image acquisition time of at least 2 min prior to storing all data and collecting them for off-line analysis. Finally, off-line analysis (Koninklijke Philips N.V., Amsterdam, The Netherlands) was used to analyze the enhancement time of the lesion and its surrounding tissue, as well as the enhancement characteristics of the lesion. The former included access speed, peak time and subsidence speed, whereas the latter included the access speed of the contrast agent into the nodule, the distribution of the contrast agent following access into the nodule, possible annular peripheral enhancement around the nodule, and the degree of clearness of the nodule boundaries.
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. The indices of SWE were compared with the t-test and the indices of CEUS were compared with Kappa analysis. A receiver operating characteristic curve (ROC) discriminating between benign and malignant thyroid nodules was drawn according to Young's modulus for each thyroid nodule. The optimal cut-off point was then determined, and the critical point and area under the curve were calculated. Sensitivity, specificity and accuracy of the SWE cut-off for the diagnosis of thyroid cancer were also calculated. Differences between benign and malignant thyroid nodules contrast modes were subjected to the χ2 test to identify the sensitivity, specificity and accuracy of CEUS in discriminating malignant thyroid nodules. The sensitivity, specificity and accuracy of 2DUS, SWE and the combined use of CEUS and SWE in the diagnosis of thyroid nodules were compared and tested using the χ2 difference test. P<0.05 was considered to indicate statistically significant differences.
Results
Surgical histopathology
Of the 253 cases (319 nodules), the pathological results showed a total of 136 malignant nodules. In terms of pathological type, there were 107 papillary, 13 follicular, 11 medullary, 4 undifferentiated and 1 squamous cell carcinoma. The pathological types of the 183 benign nodules included 135 cases of nodular goiter, of which 9 exhibited calcifications, 3 coincided with chronic lymphocytic thyroiditis, 4 exhibited follicular dysplasia, 3 were adenomatous hyperplastic nodules, 5 exhibited hemorrhage and 7 exhibited cystic degeneration; the remaining cases included 38 adenomas, 7 cases of chronic lymphocytic thyroiditis and 3 cases of subacute thyroiditis.
Diagnostic characteristics of benign and malignant thyroid nodules using 2DUS
For the 253 cases (319 nodules), 2DUS was used to evaluate benign and malignant thyroid nodules according to size, internal echo, boundaries, shape and aspect ratio, and found that nodule solidity, aspect ratio ≥1, calcification diameter <2 mm, irregular shape and unclear boundaries exhibited statistically significant differences between benign and malignant thyroid nodules (P<0.05) (Tables I and II; Fig. 1). Conventional US was associated with a sensitivity of 71.32% (97/136), a specificity of 77.04% (141/183), a positive predictive value of 69.78% (97/139), a negative predictive value of 78.33% (141/180), an accuracy of 74.60% (238/319) and a misdiagnosis rate of 25.4% (81/319) in the diagnosis of thyroid malignancy.
Table I.
Characteristics of 253 cases (319 thyroid nodules) diagnosed by conventional ultrasonography.
| Shape | Solidity | Aspect ratio | |||||
|---|---|---|---|---|---|---|---|
| Group | Nodule no. | Regular | Irregular | Solid | Cystic-solid | ≥1 | <1 |
| Benign | 183 | 161 | 22 | 157 | 26 | 14 | 169 |
| Malignant | 136 | 37 | 99 | 103 | 33 | 121 | 15 |
| χ2 | 40.31 | 5.22 | 211.36 | ||||
| P-value | 0.000 | 0.025 | 0.000 | ||||
Two-dimensional ultrasound revealed statistically significant differences (P<0.05) between benign and malignant thyroid nodules regarding shape, solidity and aspect ratio.
Table II.
Characteristics of 253 cases (319 thyroid nodules) diagnosed by conventional ultrasonography.
| Calcification diameter | Boundary | |||||
|---|---|---|---|---|---|---|
| Group | Nodule no. | <2 mm | >2 mm | Null | Clear | Unclear |
| Benign | 183 | 17 | 9 | 157 | 166 | 17 |
| Malignant | 136 | 35 | 4 | 97 | 35 | 101 |
| χ2 | 16.63 | 141.31 | ||||
| P-value | 0.000 | 0.000 | ||||
Two-dimensional ultrasound revealed statistically significant differences (P<0.05) between benign and malignant thyroid nodules regarding calcification diameter and boundaries.
Figure 1.
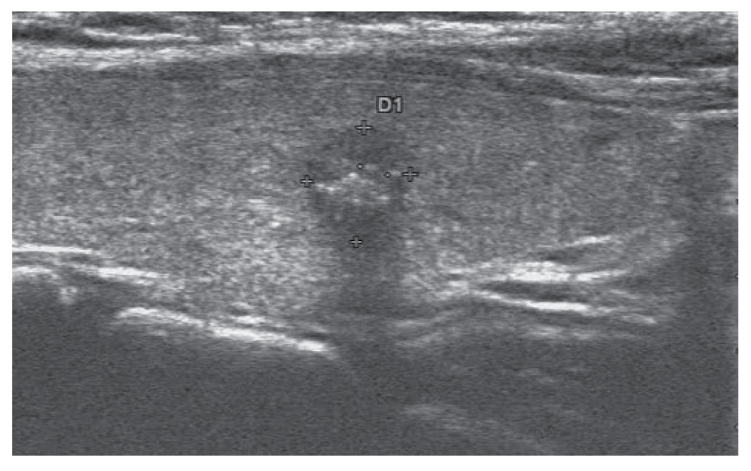
Characteristics of a malignant thyroid nodule on two-dimensional sonogram, which include solidity, hypoechogenicity, irregular boundary, aspect ratio >1 and microcalcifications.
Diagnostic characteristics of benign and malignant thyroid nodules using real-time SWE
The mean elasticity ± SD of benign and malignant thyroid nodular lesions was 49.34±23.22 and 17.99±19.55 kPa, respectively. The paired t-test of independent measurement of the same nodule by two well-trained sinologists exhibited a significance of >0.05. The differences in the SWE value between benign and malignant nodules were found to be statistically significant (t=11.932, P=0.000). ROC curves were drawn for benign and malignant thyroid nodules, in which the area under the curve was 0.77 (95% confidence interval: 0.724–0.825) and the optimal threshold was 27.65 kPa (Fig. 2). A total of 144 thyroid nodules ≥27.65 kPa were diagnosed as malignant, and 175 nodules <27.65 kPa were diagnosed as benign. Compared with the pathological results, SWE exhibited a sensitivity of 84.55% (115/136) and a specificity of 84.15% (154/183), a positive predictive value of 79.86% (115/144), a negative predictive value of 88.00% (154/175), an accuracy of 84.32% (269/319) and a misdiagnosis rate of 15.68% (50/319) in the diagnosis of malignant thyroid nodules.
Figure 2.
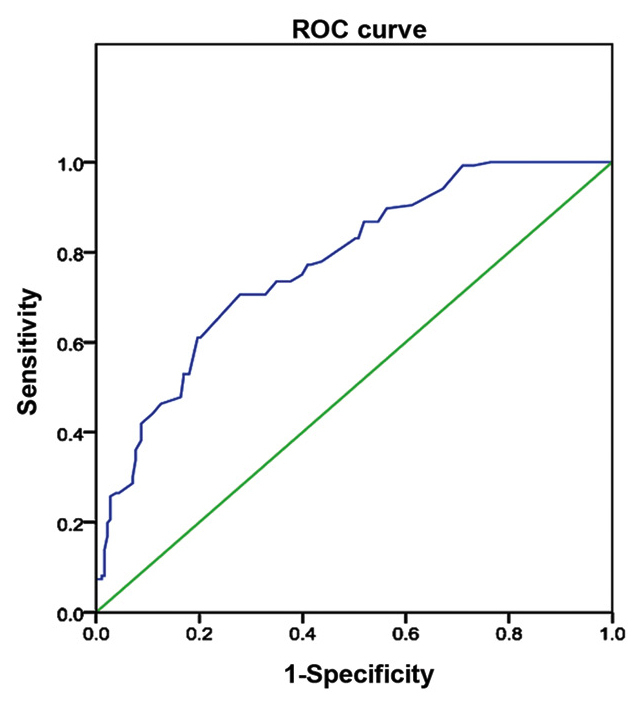
Receiver operating characteristic (ROC) curve by real shear wave elastography demonstrating the sensitivity and specificity in the diagnosis of malignant thyroid nodules; the area under the curve was 0.77.
Diagnostic characteristics of benign and malignant thyroid nodules using CEUS
CEUS was used in the diagnosis of 176 benign nodules that exhibited early high enhancement (later clearing compared with the surrounding area) following injection of the contrast medium (Fig. 4). Among these, 69 exhibited circumferential annular enhancement (Fig. 5), 165 exhibited internal homogeneous enhancement and 18 exhibited performance heterogeneous enhancement. A time-intensity curve indicated that 129 nodules were fast-filled and exhibited enhancement earlier compared with the surrounding normal glandular tissue. In particular, 38 adenomas were more highly visible, as their enhancement degree was higher compared with that of the surrounding tissue and their clearing time was slower compared with that of the remaining thyroid gland, displaying classic ‘fast-in and slow-out’ and continued high enhancement. A total of 143 thyroid nodules were diagnosed as malignant, all of which exhibited early low enhancement (Figs. 6 and 7) and quick subsidence, and were filled from the periphery towards the center, with uneven inner enhancement and less clear boundaries on US. A comparison of enhancement time phase and contrast agent distribution differences between benign and malignant thyroid nodules revealed a statistically significant difference (P<0.05) (Tables III and IV). CEUS was associated with a sensitivity of 87.5% (119/136), a specificity of 86.33% (158/183), a positive predictive value of 82.63% (119/144), a negative predictive value of 90.28% (158/175), an accuracy of 86.83% (277/319) and a misdiagnosis rate of 13.17% (42/319) in the diagnosis of benign and malignant thyroid nodules.
Figure 4.
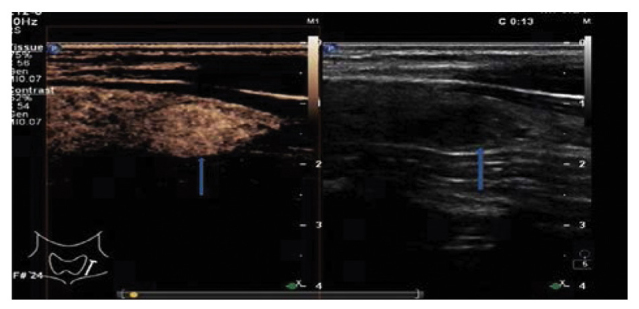
Ultrasonogram showing uniform high contrast enhancement of a benign thyroid nodule. The hypoechoic lesion of the right thyroid (arrow on the right) has an unclear boundary; on CEUS, the lesion (arrow on the left) exhibits uniform high enhancement (the lesion was pathologically diagnosed as adenomatous nodule postoperatively). CEUS, contrast-enhanced ultrasound.
Figure 5.
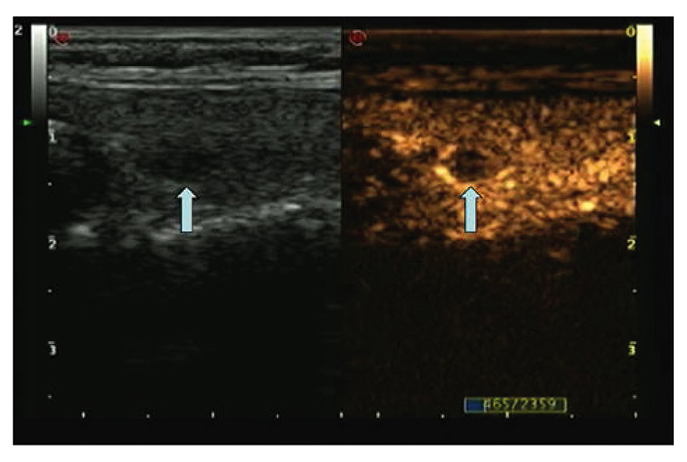
Ultrasonogram showing annular contrast enhancement of a benign thyroid nodule. The hypoechoic lesion of the right thyroid (arrow on the left) has a regular boundary; on CEUS, the lesion (arrow on the right) shows annular high enhancement (the lesion was pathologically diagnosed as adenomatous thyroid nodule with eosinophilic change). CEUS, contrast-enhanced ultrasound.
Figure 6.
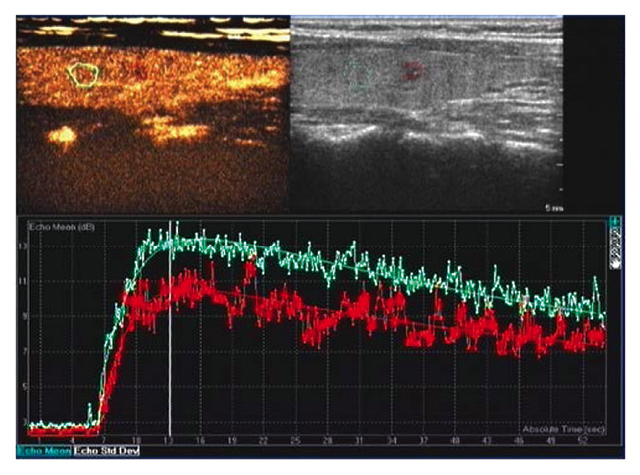
Ultrasonogram and time-intensity curve of malignant thyroid nodules. Top left panel, normal thyroid tissue sample. Top right panel, thyroid nodules. Bottom panel, time-intensity curves for the two samples. Compared with normal thyroid tissue (blue curve), thyroid nodules (red curve) exhibited low peak intensity (they were pathologically diagnosed as papillary thyroid carcinoma postoperatively).
Figure 7.
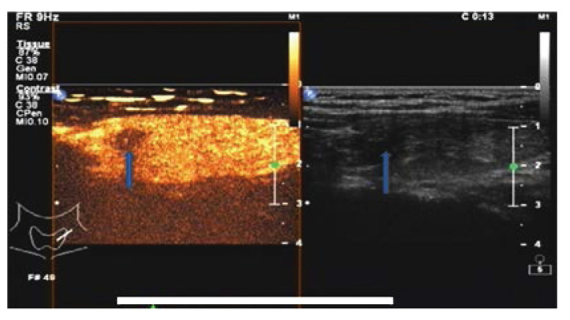
Ultrasonogram showing early low contrast enhancement of thyroid cancer. The hypoechoic lesion of the right thyroid (arrow on the right) exhibits a regular boundary. Early lesions showing low enhancement (arrow on the left). The lesions were pathologically diagnosed as papillary carcinoma postoperatively.
Table III.
Performance comparison of contrast-enhanced ultrasound in the diagnosis of benign and malignant thyroid nodules.
| Access speed | Peak time | Subsidence speed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Nodule no. | Fast | Synchronous | Slow | Earlier | Synchronous | Later | Fast | Synchronous | Slow |
| Benign | 183 | 129 | 54 | 0 | 68 | 96 | 19 | 22 | 82 | 79 |
| Malignant | 136 | 4 | 42 | 90 | 18 | 32 | 86 | 120 | 14 | 2 |
| χ2 | 206.44 | 98.99 | 182.64 | |||||||
| P-value | 0.000 | 0.000 | 0.000 | |||||||
Contrast-enhanced ultrasound revealed statistically significant differences (P<0.05) between benign and malignant thyroid nodules regarding access speed, peak time and subsidence speed.
Table IV.
Comparison of internal and boundary enhancement characteristics between benign and malignant thyroid nodules.
| Access manner | Peak intensity | Evenness | Annular enhancement | Boundary after CEUS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | No. | Centripetal | Pervasive | Low | Equal | High | Yes | No | Yes | No | Clear | Unclear |
| Benign | 183 | 11 | 172 | 11 | 51 | 121 | 165 | 18 | 69 | 114 | 141 | 42 |
| Malignant | 136 | 89 | 47 | 99 | 23 | 14 | 23 | 113 | 123 | 13 | 23 | 113 |
| χ2 | 128.00 | 162.33 | 172.93 | 90.49 | 112.90 | |||||||
| P-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |||||||
Contrast-enhanced ultrasound (CEUS) revealed statistically significant differences (P<0.05) between benign and malignant thyroid nodules regarding access manner, peak intensity, evenness and pattern of enhancement, and boundary after CEUS.
Performance comparison among 2DUS, SWE, CEUS and their combined use in the diagnosis of benign and malignant thyroid nodules (Table IV)
When comparing 2DUS, SWE, CEUS and their combined use, there were statistically significant differences in terms of sensitivity, specificity and accuracy (χ2=9.220,15.310 and 40.296; P=0.000) in the diagnosis of malignant thyroid nodules; however, there were no statistically significant differences in the diagnosis of malignant thyroid nodules between SWE and CEUS in terms of sensitivity, specificity or accuracy (χ2=0.737, P=0.542). The combined use of SWE and CEUS exhibited statistically significant differences compared with 2DUS, SWE or CEUS in terms of sensitivity, specificity and accuracy (χ2=40.296, 12.264 and 6.939; P=0.000, 0.000 and 0.005, respectively) in the diagnosis of malignant thyroid lesions. In addition, the combination of SWE and CEUS was associated with a marked increase in accuracy and a decrease in the misdiagnosis rate compared with 2DUS, SWE and CEUS in the diagnosis of malignant thyroid nodules (Fig. 8).
Figure 8.
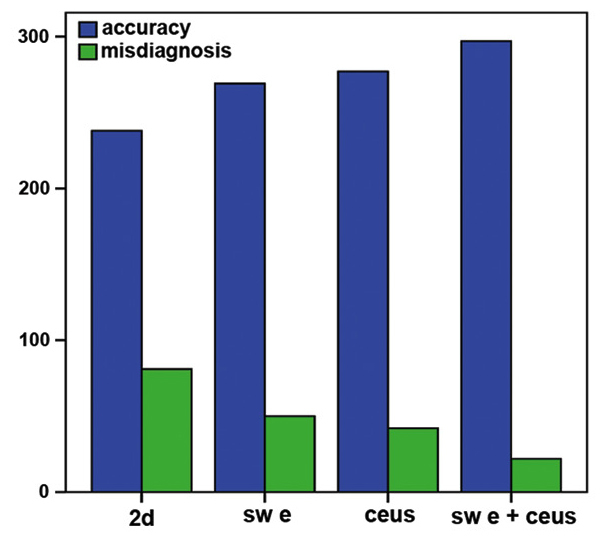
Comparison of diagnostic accuracy and misdiagnosis rate of two-dimensional ultrasound (2DUS), shear wave elastography (SWE), contrast-enhanced ultrasound (CEUS) and their combination in the investigation of thyroid malignant nodules.
Discussion
In recent years, as more individuals are subjected to thyroid examination, the detection rate of thyroid nodules has improved significantly, which is directly associated with surgical decision-making. Conventional 2DUS detects thyroid nodules and determines their nature through observation of their internal echo, boundaries, shape, calcifications and growth manner (aspect ratio). For example, malignant thyroid nodules are characterized by low echogenicity, unclear boundaries, irregular shape, internal calcification (particularly microcalcifications with a diameter of <2 mm) and ‘vertical egg type’ growth with an aspect ratio of ≥1. Although 2DUS is able to discriminate between benign and malignant thyroid nodules, its sensitivity of 71.32% (97/136), specificity of 77.04% (141/183) and accuracy of 74.60% (238/319) are relatively lower and more dependent on the examiner's experience.
SWE is a type of elastography. By virtue of acoustic radiation force impulse imaging and shear wave propagation, SWE uses the probe to launch a series of pulse waves towards the region of interest to display and quantify the hardness of tissues by generating shear waves and measuring Young's modulus. The essence of Young's modulus is the ratio of stress and strain, and its value is proportional to the square of the shear wave speed. Within the elastic limit of a target, the value is able to reflect tissue elasticity. Therefore, this method has more advantages in terms of objectivity and replicability, and has become a focus of research (6–8). Normal thyroid tissues exhibit lower hardness, which is subject to change when autoimmune or other pathogenic processes alter the thyroid cells and tissues. As thyroid tumors consist of more histological solid components but less intercellular substance and have a tendency for pervasive growth, they exhibit greater hardness and smaller elasticity upon palpation, with a higher Young's modulus. This study demonstrated that the Young's modulus of malignant nodules is higher compared with that of benign nodules, and the difference was statistically significant (t=11.932, P<0.05). Taking 29.52 kPa as the threshold, those nodules with a value of ≥29.52 kPa were diagnosed as malignant. SWE exhibited a sensitivity of 84.55% (115/136), a specificity of 84.15% (154/183) and an accuracy of 84.32% (269/319) in the diagnosis of malignant thyroid nodules, which was significantly higher compared with that of 2DUS. This suggests that real-time SWE technology is of great clinical value in the diagnosis of benign and malignant thyroid nodules. However, this technique is dependent upon the nodule, as well as the experience of the examiner; therefore, some cases may be misdiagnosed.
CEUS has emerged as a major advance in the field of medical US over the past decade. CEUS is a technology that enables real-time observation of the status of the lesion and the blood perfusion of its adjacent tissues through intravenous injection of a US contrast agent under low mechanical index. CEUS has been widely used in the examination of the liver, uterus, prostate and other organs (9,10). With the upgrading of superficial instrumentation and development of imaging techniques, clinical applications of thyroid contrast US are also increasing (11–21). In this study, the imaging of benign thyroid nodules shows that the contrast agent enters the lesions and peaks earlier or synchronically compared with the surrounding tissue, whereas its subsidence occurs more slowly or synchronically compared with the surrounding normal tissue. Furthermore, benign thyroid nodules characteristically exhibit even internal echo, clear boundaries, and annular, even and high enhancement (13–15,17,18); by contrast, malignant nodules display ‘slow access and quick subsidence’, early low enhancement (13–15,17–19), non-homogeneous distribution of the contrast agent to the center and the periphery, as well as unclear boundaries. Analysis of the differences between benign and malignant nodules on contrast imaging shows that they are mainly associated with the growth pattern of the nodules.
Benign nodules, such as thyroid adenoma (22), mainly grow in an expansive manner, gradually pushing the arteries and veins towards the periphery of the tumor, with continuous growth of new capillaries as new branches are needed. Therefore, the contrast agent reaches the center of nodule slower compared with the normal surrounding tissues. Likewise, the clearance of contrast agent is equally slow, displaying a ‘fast-in and slow-out’ imaging pattern. With nodular goiter and subacute thyroiditis (23), visible peripheral enhancement maybe observed, as they are subject to repeated stimulation, which renders the thyroid goitrous and its glands hyperplastic or atrophic. Moreover, the interior of the gland in goiter and subacute thyroiditis may suffer from necrosis due to a lack or shortage of blood supply. While some cases only exhibit continued low enhancement or no enhancement, the majority exhibit a tendency of ‘fast in and slow-out’ enhancement, which poses a risk for misdiagnosis.
The vascular structure of malignant nodules is very complex and often dense at the periphery, but relatively sparse in the central region, which explains why the blood supply of malignant nodules exhibits centripetal enhancement. The invasive growth of tumor tissue damages its surrounding tissue and new blood vessels, causing thrombus formation, arteriovenous fistulas and uneven distribution of blind-ended vessels. Larger malignant nodules may develop internal bleeding and necrosis, with coexistence of local abundance or shortage of blood supply. In addition, the poor differentiation of vascular endothelial cells, lack of muscle and nerve support, vascular dysfunction, continued destruction of tumor cells, shortage of new blood vessels, particularly lack of blood supply due to insufficient neovascularization in smaller nodules, may lead to low enhancement of the contrast agent (14–15,23). CEUS has a sensitivity of 87.5% (119/136), a specificity of 86.33% (158/183) and an accuracy of 86.83% (294/319) in the diagnosis of benign and malignant thyroid nodules.
Although CEUS exhibits high sensitivity and specificity, there remains a missed diagnosis rate of 12.5% (17/136) and a misdiagnosis rate of 13.67% (25/183). Regarding the 17 missed cases, the present study found that the majority of papillary thyroid tumors exhibit low enhancement and early low enhancement, but there are certain cases of equal enhancement (n=14) and high enhancement (n=3). Further pathological analysis confirmed 9 papillary carcinomas, 5 medullary carcinomas and 3 follicular carcinomas. The missed diagnosis may be attributed to the strong aggressiveness and rapid growth of the malignant part of the thyroid, as is suggested by adequate internal blood supply on contrast imaging. Of the 25 misdiagnosed cases of low-enhancing nodules, 18 were nodular goiter, 3 were chronic lymphocytic thyroiditis, 1 was a nodular goiter with degeneration (fibrosis, collagenous changes, calcification and hemorrhage) and 3 were subacute thyroiditis. Misdiagnosis is closely associated with the enhancement mode and pathological changes of the thyroid nodules. Benign thyroid nodules are prone to hemorrhage, cystic degeneration and calcification, which may lead to pathological structural changes. When examined by CEUS, they exhibit low enhancement, no enhancement or local non-enhancement; thus, the enhancement pattern may vary. However, CEUS has a good sensitivity in displaying internal nodular hemorrhage, cystic degeneration and necrotic areas.
Diagnosis using SWE and CEUS in the examination of malignant thyroid nodules shows no statistically significant difference in terms of sensitivity, specificity or accuracy. Nevertheless, SWE may demonstrate the hardness of nodules, whereas CEUS may illustrate blood supply, which adds information to the two-dimensional image of the nodules and evidence regarding the nature of the nodule. The combined use of SWE and CEUS was able to significantly increase the sensitivity, specificity and accuracy of diagnosis compared with 2DUS, SWE and CEUS (P<0.05), suggesting that this combination enhances the credibility of the diagnosis of malignant thyroid nodules.
In conclusion, compared with 2DUS, SWE and CEUS, the combined use of the latter two exhibits higher sensitivity, specificity and accuracy in the diagnosis of malignant thyroid nodules and the differences are statistically significant. However, two-dimensional inspection should not be omitted and, as the combination significantly increases sensitivity, specificity and accuracy in the diagnosis of malignant nodules, it maybe used as a follow-up step for further diagnosis of suspicious nodules, as it helps improve the reliability and reduce the rate of missed diagnosis. In case of diagnostic inconsistencies between CEUS and SWE, further fine-needle aspiration biopsy maybe applied to improve diagnostic accuracy.
Figure 3.
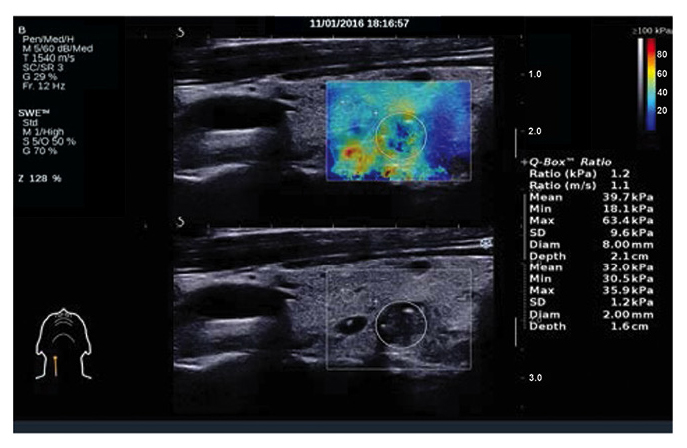
Malignant thyroid nodules on shear wave elastography. Young's modulus, 39.7 kPa.
Table V.
Performance comparison among 2DUS, SWE, CEUS and their combined use in the diagnosis of benign and malignant thyroid nodules (%).
| Pathological result | ||||||||
|---|---|---|---|---|---|---|---|---|
| Method | Type | Benign | Malignant | Sensitivity | Specificity | PPV | NPV | Accuracy |
| 2DUS | Benign | 141 | 39 | 71.32 | 77.04 | 69.78 | 78.33 | 74.60 |
| Malignant | 42 | 97 | ||||||
| SWE | Benign | 154 | 21 | 84.55a | 84.15a | 79.86a | 88.00a | 84.32a |
| Malignant | 29 | 115 | ||||||
| CEUS | Benign | 158 | 17 | 87.5a | 86.33a | 82.63a | 90.28a | 86.83a |
| Malignant | 25 | 119 | ||||||
| SWE+CEUS | Benign▼ | 170 | 9 | 93.38a,b | 92.89a,b | 90.71a,b | 94.97a,b | 93.10a,b |
| Malignant● | 13 | 127 | ||||||
Compared with conventional 2DUS, P<0.05.
Compared with SWE and CEUS, P<0.05. ▼, positive results for one or both methods; ●, negative results for both methods; SWE, shear wave elastography; CEUS, contrast-enhanced ultrasound; 2DUS, two-dimensional ultrasound; PPV, positive predictive value; NPV, negative predictive value.
References
- 1.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, Mandel SJ, Mazzaferri EL, McIver B, Pacini F, et al. Revised American thyroid association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 2.Pellegriti G, Frasca F, Regalbuto C, Squatrito S, Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Henrichsen TL, Reading CC. Thyroid ultrasonography. Part 2: Nodules. Radiol Clin North Am. 2011;49:417–424. doi: 10.1016/j.rcl.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Zeng RC, Zhang W, Gao EL, Cheng P, Huang GL, Zhang XH, Li Q. Number of central lymph node metastasis for predicting lateral lymph node metastasis in papillary thyroid microcarcinoma. Head Neck. 2014;36:101–106. doi: 10.1002/hed.23270. [DOI] [PubMed] [Google Scholar]
- 5.Sherman SI, Angelos P, Ball DW, Beenken SW, Byrd D, Clark OH, Daniels GH, Dilawari RA, Ehya H, Farrar WB, et al. Thyroid carcinoma. J Natl Compr Canc Netw. 2005;3:404–457. doi: 10.6004/jnccn.2005.0021. [DOI] [PubMed] [Google Scholar]
- 6.Gweon HM, Youk JH, Son EJ, Kim JA. Visually assessed colour overlay features in shear wave elastography for breast masses: Quantification and diagnostic performance. Eur Radiol. 2013;23:658–663. doi: 10.1007/s00330-012-2647-3. [DOI] [PubMed] [Google Scholar]
- 7.Ferraioli G, Tinelli C, Zicchetti M, Above E, Poma G, Di Gregorio M, Filice C. Reproducibility of real-time shear wave elastography in the evaluation of liver elasticity. Eur J Radiol. 2012;81:3102–3106. doi: 10.1016/j.ejrad.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 8.Woo S, Kim SY, Cho JY, Kim SH. Shear wave elastography for detection of prostate cancer: A preliminary study. Korean J Radiol. 2014;15:346–355. doi: 10.3348/kjr.2014.15.3.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pop CM, Mihu D, Badea R. Role of contrast-enhanced ultrasound (CEUS) in the diagnosis of endometrial pathology. Clujul Med. 2015;88:433–437. doi: 10.15386/cjmed-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Q, Lv F, Luo Y, Song Q, Xu Q, Su Y, Tang Y, Tang J. Contrast-enhanced ultrasound for evaluation of renal trauma during acute hemorrhagic shock: A canine model. J Med Ultrason (2001) 2015;42:199–205. doi: 10.1007/s10396-014-0601-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim BK, Choi YS, Kwon HJ, Lee JS, Heo JJ, Han YJ, Park YH, Kim JH. Relationship between patterns of calcification in thyroid nodules and histopathologic findings. Endocr J. 2013;60:155–160. doi: 10.1507/endocrj.EJ12-0294. [DOI] [PubMed] [Google Scholar]
- 12.Bartolotta TV, Midiri M, Galia M, Runza G, Attard M, Savoia G, Lagalla R, Cardinale AE. Qualitative and quantitative evaluation of solitary thyroid nodules with contrast-enhanced ultrasonography: Initial results. Eur Radiol. 2006;16:2234–2241. doi: 10.1007/s00330-006-0229-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Jiang YX, Liu JB, Yang M, Dai Q, Zhu QL, Gao P. Utility of contrast-enhanced ultrasound for evaluation of thyroid nodules. Thyroid. 2010;20:51–57. doi: 10.1089/thy.2009.0045. [DOI] [PubMed] [Google Scholar]
- 14.Ma JJ, Ding H, Xu BH, Xu C, Song LJ, Huang BJ, Wang WP. Diagnostic performances of various gray-scale, color Doppler, and contrast-enhanced ultrasonography fidings in predicting malignant thyroid nodules. Thyroid. 2014;24:355–363. doi: 10.1089/thy.2013.0150. [DOI] [PubMed] [Google Scholar]
- 15.Cantisani V, Consorti F, Guerrisi A, Guerrisi I, Ricci P, Di Segni M, Mancuso E, Scardella L, Milazzo F, D'Ambrosio F, Antonaci A. Prospective comparative evaluation of quantitative-elastosonography (Q-elastography) and contrast-enhanced ultrasound for the evaluation of thyroid nodules: Preliminary experience. Eur J Rsdiol. 2013;82:1892–1898. doi: 10.1016/j.ejrad.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Moon WJ, Jung SL, Lee JH, Na DG, Baek JH, Lee YH, Kim J, Kim HS, Byun JS, Lee DH. Thyroid Study Group, Korean Society of Neuro- and Head and Neck Radiology: Benign and malignant thyroid nodules: US differentiation-multicenter retrospective study. Radiology. 2008;247:762–770. doi: 10.1148/radiol.2473070944. [DOI] [PubMed] [Google Scholar]
- 17.Hong YR, Yan CX, Mo GQ, Luo ZY, Zhang Y, Wang Y, Huang PT. Conventional US, elastopraphy, and contrast enhanced US features of papillary thyroid microcarcinoma predict cental compartment lymph node matastases. Sci Rep. 2015;5:7748. doi: 10.1038/srep07748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oyedeji F, Giampoli E, Ginat D, Dogra V. The sonographic appearance of benign and malignant thyroid diseases and their histopathology correlate:Demystifying the thyroid nodule. Ultrasound Q. 2013;29:161–178. doi: 10.1097/RUQ.0b013e31829a573e. [DOI] [PubMed] [Google Scholar]
- 19.Li F, Luo H. Comparative study of thyroid puncture biopsy guided by contrast-enhanced ultrasonography and conventional ultrasonography. ExpTher Med. 2013;5:1381–1384. doi: 10.3892/etm.2013.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giusti M, Orlandi D, Melle G, Massa B, Silvestri E, Minuto F, Turtulici G. Is there a real diagnostic impact of elastosonography and contrast-enhanced ultrasonography in the management of thyroid nodules? J Zhejiang Univ Sci B. 2013;14:195–206. doi: 10.1631/jzus.B1200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nemec U, Nemec SF, Novotny C, Weber M, Czerny C, Krestan CR. Quantitative evaluation of contrast-enhanced ultrasonography after intravenous administration of a microbubble contrast agent for differentiation of benign and malignant thyroid nodules: Assessment of diagnostic accuracy. Eur Radiol. 2012;22:1357–1365. doi: 10.1007/s00330-012-2385-6. [DOI] [PubMed] [Google Scholar]
- 22.Jiang J, Huang L, Zhang H, Ma W, Shang X, Zhou Q, Gao Y, Yu S, Qi Y. Contrast-enhanced sonography of thyroid nodules. J Clin Ultrasound. 2015;43:153–156. doi: 10.1002/jcu.22240. [DOI] [PubMed] [Google Scholar]
- 23.Friedrich-Rust M, Sperber A, Holzer K, Diener J, Grünwald F, Badenhoop K, Weber S, Kriener S, Herrmann E, Bechstein WO, et al. Real-time elastography and contrast-enhanced ultrasound for the assessment of thyroid nodules. Exp Clin Endocrinol Diabetes. 2010;118:602–609. doi: 10.1055/s-0029-1237701. [DOI] [PubMed] [Google Scholar]


