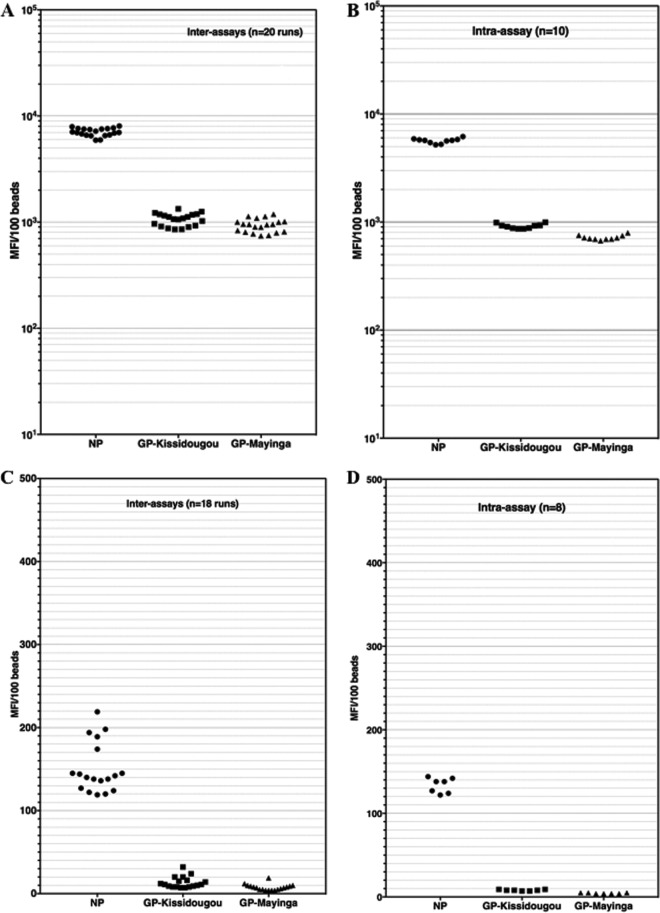
FIG 1.
Interassay and intra-assay variabilities of EBOV-positive and -negative samples in the Luminex assay. An Ebola virus survivor's sample and a negative plasma sample were used to evaluate intra-assay and interassay variability of the novel Luminex assay. Plasma samples were tested at a 1/1,000 dilution in assay buffer and repeated 8 to 10 times within the same run (intra-assay variability [B and D]) or tested in 20 different runs over 3 weeks (interassay variability [A and C]). Panels A and B illustrate these variabilities for an EBOV-positive sample, while panels C and D illustrate the variabilities of an Ebola virus-negative sample.