TABLE 2.
Homogeneity of MICs obtained with supplemented CAMHB by using two different incubation times
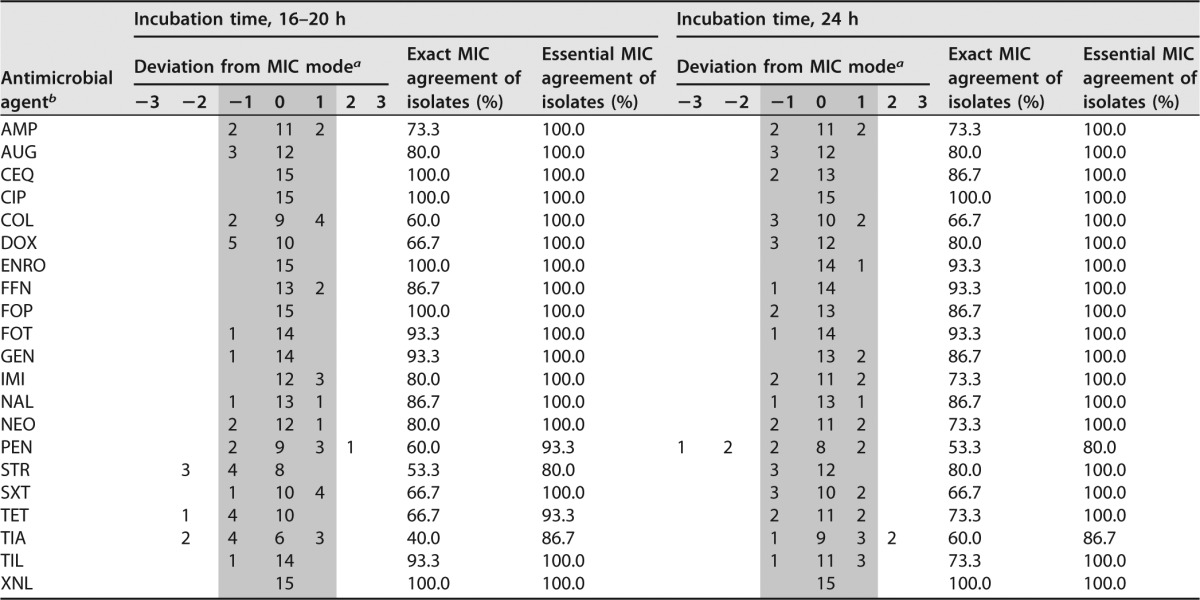
a Data fulfilling the criteria of the essential MIC agreement are in the gray area.
b AMP, ampicillin; AMC, amoxicillin-clavulanic acid; CIP, ciprofloxacin; COL, colistin; CQN, cefquinome; DOX, doxycycline; ENRO, enrofloxacin; FFN, florfenicol; FOP, cefoperazone; FOT, cefotaxime; GEN, gentamicin; IMI, imipenem; MAR, marbofloxacin; NAL, nalidixic acid; NEO, neomycin; PEN, penicillin; STR, streptomycin; SXT, trimethoprim-sulfamethoxazole; TET, tetracycline; TIA, tiamulin; TIL, tilmicosin; XNL, ceftiofur.
