Figure 2.
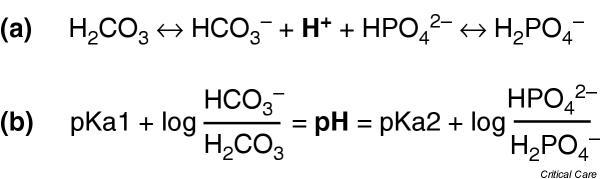
The isohydric principal expressed in (a) the law of mass action form and (b) the Henderson–Hasselbalch form. Because all weak acids in a solution are in equilibrium with a single pool of hydrogen ions, the ratio of any of the conjugate anion and its undissociated acid will be able to describe the pH.
