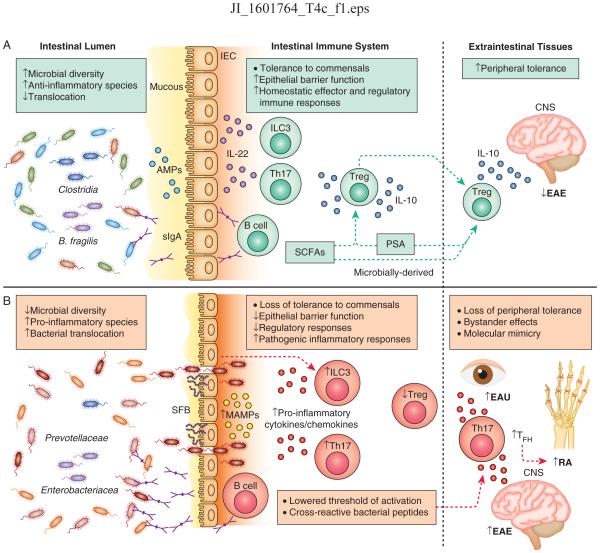
Figure 1. Host-microbiota interactions underlie homeostasis and inflammation in the intestine and extraintestinal tissues.
A) At homeostasis, gut bacteria are compartmentalized within the lumen through exclusion by the mucous, neutralization by anti-microbial peptides (AMPs) produced by intestinal epithelial cells (IECs), and release of secretory IgA (sIgA) from intestinal resident B cells. In response to various cues, ILC3s and Th17 cells in the intestine produce IL-22 which acts on IECs to promote compartmentalization of the microbiota. Regulatory T cells (Treg) produce IL-10 and are induced by microbially-derived short chain fatty acids (SCFAs) and polysaccaride A (PSA) or by the bacterial species B. fragilis and Clostridia. Intestinal activation of Tregs can protect against neuroinflammation in the central nervous system (CNS) during experimental autoimmune encephalitis (EAE). B) During chronic intestinal inflammation, loss of intestinal barrier function results in bacterial translocation across the epithelium, release of commensally derived microbe-associated molecular patterns (MAMPs), pro-inflammatory cytokine and chemokine activation, and Th17 and B cell responses. Specific bacteria exacerbate intestinal inflammation including Prevotellaceae, Enterobacteriacea and the Th17-inducing segmented filamentous bacteria (SFB). Loss of tolerance to self antigens can occur due to lowered thresholds for auto-activation (‘bystander effect’) that can mediate autoimmunity in extraintestinal tissues. Bystander effects initiate and exacerbate Th17-mediated inflammation in mouse models of EAE and rheumatoid arthritis (RA). In RA, Th17 and TFH responses aid in auto-antibody production in secondary lymph nodes. Licensing of cross-reactive T cell responses that recognize microbially-derived peptides and react to self-peptides can also initiate autoimmunity in extraintestinal tissues, exemplified in a mouse model of experimental autoimmune uveitis (EAU).