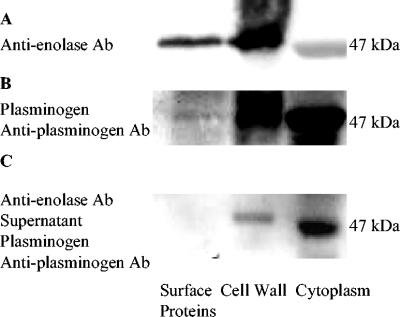
FIG. 1.
Plasminogen binding to S. mutans enolase. (A) Electrophoretically separated cell surface, cell wall, and cytoplasmic samples were incubated with rabbit anti-enolase antibody (Ab) (1:300) and anti-rabbit IgG-peroxidase (1:500). (B) Separated surface protein, cell wall, and cytoplasm samples were incubated with plasminogen (0.02 mg/ml), goat anti-plasminogen, and anti-goat IgG-peroxidase (1:500). (C) Surface protein, cell wall, and cytoplasm samples were incubated with blocking rabbit anti-enolase antibody (1:300) and centrifuged. The supernatants of those samples were isolated by SDS-PAGE and transferred to a PVDF membrane, which was incubated with plasminogen (0.02 mg/ml), goat anti-plasminogen, and anti-goat IgG-peroxidase (1:500). The binding activity of enolase with plasminogen was decreased by blocking rabbit anti-enolase antibody.