Abstract
Background
To our knowledge, no reliable clinical prediction rule (CPR) for identifying bacteremia in hemodialysis (HD) patients has been established. The aim of this study was to develop a CPR for bacteremia in maintenance HD patients visiting the outpatient department.
Methods
This multicenter cohort study involved consecutive maintenance HD patients who visited the outpatient clinic or emergency room of seven Japanese institutions between August 2011 and July 2013. The outcome measure was bacteremia diagnosed based on the results of blood cultures. The candidate predictors for bacteremia were extracted through a literature review. A CPR for bacteremia was developed using a coefficient-based multivariable logistic regression scoring method, and calibration was performed. The test performance was then assessed for the CPR.
Results
Of 507 patients eligible for the study, we analyzed the 293 with a complete dataset for candidate predictors. Of these 293 patients, 48 (16.4%) were diagnosed with bacteremia. At the conclusion of the deviation process, body temperature ≥ 38.3°C, heart rate ≥ 125 /min, C-reactive protein ≥ 10 mg/dL, alkaline phosphatase >360 IU/L, and no prior antibiotics use within the past week were retained and scored. The CPR had a good fit for the model on calibration. The AUC of the CPR was 0.76, and for score CPR ≥ 2, the sensitivity and specificity were 89.6% and 51.4%, respectively.
Conclusions
We established a simple CPR for bacteremia in maintenance HD patients using routinely obtained clinical information in an outpatient setting. This model may facilitate more appropriate clinical decision making.
Introduction
Bacteremia is a common and serious condition worldwide and associated with a high mortality [1]. This condition is a particularly thorny problem in hemodialysis (HD) patients, since the morbidity and mortality of bacteremia in this population are extremely high compared to the general population [2–10]. Therefore, appropriate diagnosis and treatment are of great import in HD patients, as their outcomes can be quite poor.
Several clinical prediction rules (CPRs) with excellent predictive ability for bacteremia in the general population have been developed in recent years [11–14]. However, these CPRs include predictors that are difficult to apply to HD patients, such as serum creatinine, blood urea nitrogen, and electrolyte concentration, since these variables are known to be greatly affected by dialysis treatment. To our knowledge, no CPRs for bacteremia in HD patients have yet been established, a marked unmet need.
Two major differences have been noted with respect to bacteremia infection between maintenance HD patients and the general population. First, the incidence of bacteremia is much higher in maintenance HD patients than in the general population, as mentioned above. Previous cohort studies have shown that the incidence of bacteremia in maintenance HD patients is 10.40–18.98 per 100 person-years [2–7, 15], which is higher than the incidence of 0.216 per 100 person-years in the general population [1]. Further, the annual mortality due to bacteremia in HD patients is 100–300 times that in the general population. Even after adjustment for age, race, sex, diabetes status, and record errors, the mortality due to bacteremia is still 50 times higher in these patients than in the general population [8–10]. As such, bacteremia is considered a disease that is both extremely common and associated with serious outcomes in maintenance HD patients. Second, the pathogen type and etiology of bacteremia in maintenance HD patients differs from those commonly observed in the general population. The most frequently identified pathogen of bacteremia in the general population is Escherichia coli (E. coli), accounting for 22%-54% of cases [1, 16–21], with the urinary tract reportedly the most common route of infection, followed by the respiratory and gastrointestinal tracts [22]. In contrast, in maintenance HD patients, the most frequently identified pathogen of bacteremia is Staphylococcus aureus, accounting for 27%-39% of cases [6, 23, 24], with the most frequent routes of infection reportedly transdermal and trans-catheter sites.
Despite the substantial differences in the pathology and prognosis of bacteremia between HD patients and the general population, no CPRs have yet been established specifically targeting this at-risk group. In the present study, we developed and validated a new CPR for bacteremia specifically for maintenance HD patients.
Materials and Methods
Study Design
We conducted a multicenter retrospective cohort study of maintenance HD patients at six tertiary-care institutions, all of which receive patients on an emergency basis and all of which provide primary, secondary, and tertiary care; and one secondary-care institution that receives patients on an emergency basis and provides both primary and secondary care. All seven institutions are teaching hospitals. The present study was approved by the ethics committee of St. Marianna Medical University (No. 2713), and the study was conducted in accordance with the ethical standards of the Declaration of Helsinki. In the present study, the Department of Nephrology and Hypertension, St. Marianna University School of Medicine, connected anonymous data from the participating facilities. In addition, since all of the patient information analyzed in this study was retrospective, the consent of participants was not obtained.
In the present study, the Department of Nephrology at Chubu Rosai Hospital compiled anonymous data from the participating facilities. In addition, since all patient information analyzed in this study was retrospective, participants’ consent was not obtained. The study results are reported in accordance with the Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD) statement [25].
Participants
The inclusion criteria of the present study were as follows: consecutive HD patients who visited the outpatient department or emergency room between August 2011 and July 2013 and had blood drawn for cultures within 48 h from their initial arrival at the hospital. The exclusion criteria of this study were follows: under 18 years old; low frequency of HD (<1 time per week); combination of peritoneal dialysis; less than 2 weeks from the introduction of HD; and hospitalized patients referred from another hospital.
Among the participants who met the eligibility criteria, those with a complete dataset for candidate predictors were assigned to the derivation set. The set for internal validation was extracted using the bootstrap method.
We estimated that more than 10 cases with the outcome for each potential predictor in a multivariate model were needed to develop a clinical prediction model, based on common practice. Since we were attempting to create a CPR consisting of 5 item predictors, 50 outcomes were estimated to be necessary. The proportion of bacteremia in our study participants were estimated to 16%, based on the findings of previous Japanese studies conducted in the general population, which is thought to have a lower proportion of bacteremia than an HD population. We therefore estimated the required sample size to be a minimum of 320.
Outcome Measures
The primary outcome measure was bacteremia diagnosed is based on the results of blood cultures at the time of the patient`s visit. The diagnosis of bacteremia was made when any bacteria not attributed to contamination were detected in a blood culture. Contamination was defined as follows: cases where only 1 of 2 sets of culture bottles was positive, or cases with detection of certain species of bacteria, such as diphtheroids, Bacillus sp., Propionibacterium sp., micrococci, Corynebacterium sp., and coagulase-negative staphylococci. Finally, an external consensus panel of two physicians well-trained in infectious diseases determined whether a culture was contaminated or not based on the above definitions and their clinical expertise.
Candidate Predictors
Exhaustive variables already known to be predictors for bacteremia were selected from CPRs analyzed in an existing systematic review [11] and extracted by adding a search period through April 1, 2016, using the same search formula of MEDLINE via PubMed as a systematic review by Eliakim et al., as follows: ((predict[All Fields] OR predicting[All Fields] OR prediction[All Fields]) AND (("bacteraemia"[All Fields] OR "bacteremia"[MeSH Terms] OR "bacteremia"[All Fields]) OR (("blood"[Subheading] OR "blood"[All Fields] OR "blood"[MeSH Terms]) AND ("rivers"[MeSH Terms] OR "rivers"[All Fields] OR "stream"[All Fields]) AND ("infection"[MeSH Terms] OR "infection"[All Fields])))) AND ("2014/10/01"[PDAT]: "2016/04/01"[PDAT]) (12–14). The candidate predictors were then selected from among these exhaustive predictors through consultation with two reviewers, each of whom had more than 10 years’ experience as nephrologists, based on the predictors’ usefulness in clinical practice in maintenance HD patients.
The final selected predictors were as follows: age, vital signs at the time of visit (body temperature, systolic blood pressure, pulse rate, percutaneous oxygen saturation, Glasgow Coma Scale [GCS]), antibiotics use within one week from hospital visit, patient’s complaints (chill, nausea, focal abdominal signs), and laboratory data at hospital visit (white blood cell [WBC] count, platelet count, serum albumin, serum alkaline phosphatase [ALP], C-reactive protein [CRP]).
Specific Predictors for the HD Population
In addition to the candidate predictors, we identified several further predictors for bacteremia that are specific to the HD population. Variables considered to be related to bacteremia were selected by referencing the existing literature and conducting multivariate regression with clinical expertise. We then selected those variables readily available in a general clinical setting as the specific predictors from among the available variables. Ultimately, non-arteriovenous fistula (non-AVF) use as vascular access [26–29] was identified as a predictor specific to HD patients.
Statistical Analysis
Descriptive statistics
We analyzed the predictors and the outcome as well as other clinical information, including gender, HD vintage, cause of chronic kidney disease (CKD), and pathogens of bacteremia. Continuous and categorical variables are presented as the median (quartile) and number (percentage), respectively.
Development of a prediction rule
Among the candidate predictors, the continuous variables were changed to binary variables based on the cut-off value referenced from previous studies. All of the candidate predictors were selected through stepwise backward selection with a p-value<0.05. We then analyzed the cases with complete data available for the selected predictors via this process.
To establish a CPR from the candidate predictors, a regression coefficient-based scoring method was used. First, the ratio based on each β-coefficient relative to a reference that was an intermediate value of the two variables with the smallest β-coefficient was calculated. Then, the ratio was converted to the appropriate integer. The appropriate integer was chosen from integers close to the number ensuring the highest predictive ability for bacteremia. The total score was calculated by summing the scores for each variable.
For further investigation, the modified CPR, developed by adding non-AVF as an HD-specific predictor for bacteremia to the established CPR, was scored to verify the influence of these predictors on the predictive ability for bacteremia. Calibration was performed based on the Hosmer-Lemeshow chi-square statistic [30] and the slope and intercept of the calibration plot [31] for the CPR.
Assessment of test performance
To evaluate potential cut-off scores, we computed the sensitivity, specificity, likelihood ratio, positive predictive value, and negative predictive value for the CPR.
Validation process
To validate the final model, we used a bootstrapping technique with 200 resamples [32]. The discriminatory ability of the prediction rules was assessed by the area under the receiver operating characteristic curve. All of the statistical analyses were performed using Stata version 13.1 (Stata Corp., College Station, TX, USA).
Results
Description of Study Cohort
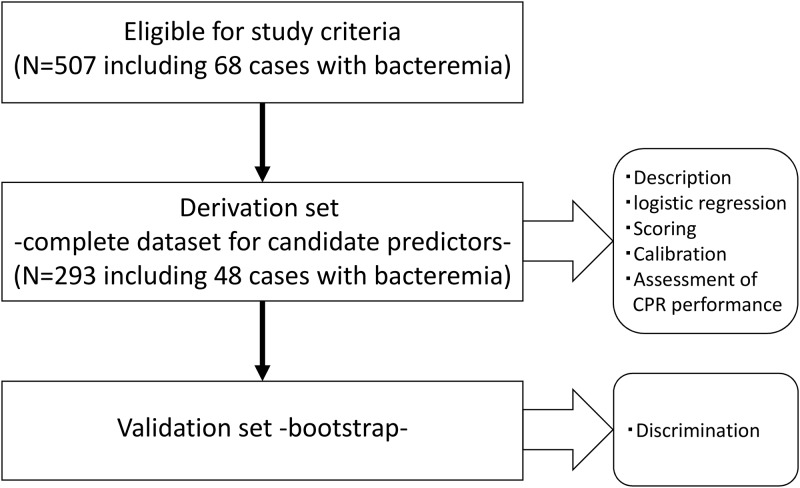
Among 507 participants with 68 cases of bacteremia who met the eligible study criteria, the 293 participants with 48 cases of bacteremia who had a complete dataset for the candidate predictors were analyzed, as shown in Fig 1. Table 1 shows the baseline characteristics of the participants. Of the 293 participants, the median age was 74 years, 66.6% were men, the most frequent cause of chronic kidney disease was diabetic nephropathy (42.0%), the most frequent route of vascular access was arteriovenous fistula (83.6%), the mean HD vintage was 61 months, and 16.4% of patients had taken antibiotics within 1 week prior to hospital visit.
Fig 1. Study flow CPR: clinical prediction rule.
Table 1. Baseline characteristics (N = 293).
| Median (quartile or %) | Median (quartile or %) | ||
|---|---|---|---|
| Sex | Medication | ||
| Male | 195 (66.6) | Steroid use | 33 (11.3) |
| Female | 98 (33.5) | Immunosuppressant use | 5 (1.7) |
| Age (years) | 74 (66, 81) | Antibiotics use within 1 week | 48 (16.4) |
| Vital signs | Symptoms | ||
| Body temperature (°C) | 37.2 (36.6, 38.1) | Chill | 13 (4.4) |
| Systolic blood pressure (mmHg) | 136 (114, 160) | Nausea | 28 (9.6) |
| Heart rate (/min) | 84 (74, 100) | Focal abdominal sign | 29 (9.9) |
| SpO2 (%) | 97 (95, 98) | Causes of CKD | |
| (FiO2) | 0.21 (0.21, 0.21) | Diabetic nephropathy | 123 (42.0) |
| GCS<15 | 45 (15.4) | Hypertensive nephrosclerosis | 61 (20.8) |
| HD vintage (months) | 61 (23, 112) | Chronic glomerulonephritis | 45 (15.4) |
| Vascular access | Others and unknown | 64 (21.8) | |
| AV fistula | 245 (83.6) | Laboratory findings | |
| AV graft | 28 (9.6) | White blood cell (/μL) | 8400 (5900, 11300) |
| Superficial artery | 16 (5.5) | Platelet count (/μL) | 14.9 (10.5, 20) |
| Permanent catheter | 4 (1.4) | Albumin (mg/dL) | 3.3 (2.9, 3.6) |
| History of bacteremia | 31 (10.6) | Alkaline phosphatase (IU/L) | 271 (212, 382) |
| Comorbidities | C-reacted protein (mg/dL) | 6.1 (1.8, 12.8) | |
| Diabetes mellitus | 131 (44.7) | Bacteremia | 48 (16.3) |
| Malignancy | 33 (11.3) |
SpO2: oxyhemoglobin saturation measured by pulse oximetry, FiO2: fraction of inspiratory oxygen, GCS: Glasgow Coma Scale, HD: hemodialysis, AV fistula: arteriovenous fistula, CKD: chronic kidney disease
Table 2 shows the pathogens associated with bacteremia in our population. The most frequent pathogen was Staphylococcus aureus, accounting for 39.6% (19 cases) of all bacteremia cases. Of these cases, 12 were Methicillin-sensitive S. aureus (MSSA), while the other 7 were Methicillin-resistant S. aureus (MRSA). Klebsiella pnuemoniae and Escherichia coli accounted for 9 and 7 cases, respectively. Among 48 cases with bacteremia, 4 were polymicrobial.
Table 2. Pathogens of bacteremia.
| Bacteria | N | Bacteria | N |
|---|---|---|---|
| Staphylococcus aureus | 19 | Ecterococcus faecalis | 1 |
| Methicillin-sensitive Staphylococcus aureus | 12 | Pseudomonas aerugiosa | 1 |
| Methicillin-resistant Staphylococcus aureus | 7 | Streptococcus salivarius | 1 |
| Klebsiella pneumoniae | 9 | Streptococcus pneumoniae | 1 |
| Escherichia coli | 7 | Streptococcus mutans | 1 |
| Coaglase-negative staphylococcus species | 5 | Parabacteroides distasonis | 1 |
| Clostridium perfringens | 2 | Helicobacter cinaedi | 1 |
| Bacteroides | 2 | Anaerobic gram-negative bacilli | 1 |
| Enterococcus faecium | 2 |
CPR
Stepwise backward selection identified body temperature ≥ 38.3°C, pulse rate ≥ 125/min, CRP ≥ 10 mg/dL, ALP ≥ 360 IU/L, and no antibiotics used within one week before the hospital visit as potential predictors. Table 3 shows the results of regression coefficient-based scoring. Our CPR ultimately included the above 5 variables with 1 point each (total 5 points). The Hosmer-Lemeshow chi-squared statistic for the CPR was 1.99 (p = 0.57), and the calibration slope (intercept) of the CPR was 0.86 (0.01). The sensitivities, specificities, and likelihood ration for possible cut-off scores in prediction rule are shown in Table 4. With a value of 2 set as the cut-off score in the CPR, the sensitivity was 89.6%, the specificity was 51.7%, the positive likelihood ratio was 1.8, the negative likelihood ratio was 0.2, and the percentage of false negatives was 3.8% (5/131).
Table 3. Multivariate analysis and scoring.
| Selected variables | Β-coefficient | 95% CI | p-value | Score |
|---|---|---|---|---|
| Body temperature ≥ 38.3°C | 1.12 | 0.34, 1.91 | <0.01 | 1 |
| Heart rate ≥ 125 /min | 1.12 | 0.01, 2.22 | 0.04 | 1 |
| CRP ≥ 10 mg/dL | 1.31 | 0.60, 2.01 | <0.01 | 1 |
| ALP > 360 IU/L | 1.05 | 0.35, 1.74 | <0.01 | 1 |
| No prior ABx within 1 w | 1.3 | 0.15, 2.45 | 0.03 | 1 |
CRP: C-reactive protein, ALP: alkaline phosphatase, ABx: antibiotics, 95% CI: 95% confidence interval
Table 4. Assessment of test performance.
| Cutoff | Total | Bacteremia | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR- (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|---|---|
| ≥1 | 278 | 48 | 100 (92.6, 100) | 6.1 (3.5, 9.9) | 1.1 (1, 1.1) | 0 | 17.3 (13, 22.2) | 100 (78.2, 100) |
| ≥2 | 162 | 43 | 89.6 (77.3, 96.5) | 51.4 (45, 57.8) | 1.8 (1.6, 2.2) | 0.2 (0.1, 0.5) | 26.5 (19.9, 34) | 96.2 (91.3, 98.7) |
| ≥3 | 54 | 22 | 45.8 (31.4, 60.8) | 86.9 (82.1, 90.9) | 3.5 (2.3, 5.5) | 0.6 (0.5, 0.8) | 40.7 (27.6, 55) | 89.1 (84.5, 92.8) |
| ≥4 | 9 | 5 | 10.4 (3.5, 22.7) | 98.4 (95.9, 99.6) | 6.4 (1.8, 22.9) | 0.9 (0.8, 1) | 55.6 (21.2, 86.3) | 84.9 (80.2, 88.8) |
| ≥5 | 0 | 0 |
LR+: positive likelihood ratio, LR-: negative likelihood ration, PPV: positive predictive value, NPV: negative predictive value, 95% CI: 95% confidence interval
Next, as shown in Table 5, the modified CPR including non-AVF as the HD-specific predictor was scored. The Hosmer-Lemeshow chi-squared statistic for the modified CPR was 2.12 (p = 0.71). The calibration slope and intercept of the CPR were 0.97 and -0.02, respectively.
Table 5. Multivariate analysis and scoring for the modified CPR.
| Variables | Β-coefficient | 95% CI | p-value | Score | |
|---|---|---|---|---|---|
| Established CPR + non-AVF | Body temperature ≥ 38.3°C | 1.12 | 0.34, 1.91 | <0.01 | 1 |
| Heart rate ≥ 125/min | 1.12 | 0.01, 2.22 | 0.04 | 1 | |
| CRP ≥ 10 mg/dL | 1.31 | 0.60, 2.01 | <0.01 | 1 | |
| ALP > 360 IU/L | 1.05 | 0.35, 1.74 | <0.01 | 1 | |
| No prior ABx within 1 w | 1.3 | 0.15, 2.45 | 0.03 | 1 | |
| Non-AVF | 1.3 | 0.15, 2.45 | 0.03 | 1 |
CPR: clinical prediction rule, CRP: c-reacted protein, ALP: alkaline phosphatase, ABx: antibiotics, 1 w: one week, AVF: arteriovenous fistula
Internal Validation—CPR
The internal validity of the CPR was verified in the participants extracted via the bootstrap method. The area under the ROC curve (AUC) of the CPR was 0.76 (95% confidence interval [CI]: 0.69, 0.82).
Internal Validation—Modified CPRs Adding HD-specific Predictors
The internal validity of the modified CPR adding non-AVF was verified in the participants extracted via the bootstrap method. The AUC of the modified CPR was 0.74 (95% CI: 0.67, 0.81).
Discussion
In this study, we developed a CPR for bacteremia in maintenance HD patients. Although, previous studies have already established CPRs for bacteremia in the general population [11–14], to our knowledge, this is the first prediction rule modified for use in maintenance HD patients. Of the two CPRs, we determined that the CPR not including non-AVF as an HD-specific predictor is the more useful of the pair for clinicians for three reasons. First, the CPR without non-AVF has better diagnostic accuracy with a higher AUC. Second, the study population of this investigation has a much higher proportion of AVF, which is the most common vascular access among Japanese HD patients. As such, a CPR with non-AVF might have low generalizability. Third, a score of ≥2 for the CPR without any HD-specific predictors had high sensitivity and a negative predictive value. We therefore named this CPR the “BAC-HD” (Body temperature ≥38.3°C, Alkaline phosphatase ≥360 IU/L, CRP ≥10 mg/dL, Heart rate ≥125/min, Drug: no prior ABx use within 1 week) score.
A BAC-HD score ≥2 with high sensitivity can be useful for ensuring that bacteremia infections, which are a common and serious issue for HD patients, are not missed. Careful observation can lead to prompt initiation of intravenous antibiotics, which reduces the mortality rate due to infection in dialysis patients.
The BAC-HD score has two major strengths. First, these criteria are considered suitable for use in actual clinical settings, since they include routine clinical data. Specifically, the BAC-HD contains two blood test items—ALP and CRP—that are relatively easy to measure in a Japanese hospital setting; these items provide are nearly as easy to measure as a Complete Blood Count. Furthermore, the present CPR is expected to be used in other facilities besides secondary medical institutions, and the ALP can be measured within 1 h in such facilities. In situations involving HD patients suspected of having bacteremia, although the body temperature, heart rate, history of antibiotic use and CRP are evaluated as a rule in general, the addition of ALP measurement may further support making clinical decisions. Second, the criteria are extremely simple, with only five items, which is few compared with the CPRs for bacteremia in the general population that mostly have more than 10 items. We believe that the simplicity of the BAC-HD score may be attributed to the relatively uniform etiology of bacteremia in HD patients compared with the general population. This simplicity should facilitate the detection of bacteremia in maintenance HD patients and encourage rapid and appropriate decision-making.
In this prediction rule, in addition to the items known to be associated with bacteremia in the general population, we incorporated non-AVF as an HD patient-specific item, which is known to be a strong risk factor for blood stream infections in maintenance HD patients [33] [34, 35]. However, the CPR with non-AVF did not show a superior predictive ability to the original CPR without non-AVF. We attribute this lack of superiority to the markedly high proportion of AVF vascular access in Japan, in contrast to the situation in the United States [36]. Indeed, even in our cohort, the proportion of AVF was 83.6%, indicating little variation, which may have diluted the contribution of non-AVF to bacteremia. Given the recent launch of the “Fistula First” awareness campaign by the National Kidney Foundation to promote the initiation of AVF vascular access in HD induction, we expect AVF access to become mainstream globally. In this context, our prediction rule is considered to be a suitable model for global HD patients in the future.
In the present study, a high ALP level was extracted as an effective predictor. A small observational study further suggested the close relationship between an extremely high ALP level and bacteremia [37]. In that study, the authors speculated that the observed extremely high ALP level was the result of bacteremia-related hepatic dysfunction. ALP is also considered to have an anti-inflammatory effect, given its dephosphorylating and lipopolysaccharide (LPS) detoxifying activity [38–40]. However, LPS is produced by gram-negative bacteria; as such, this etiology for ALP elevation cannot explain the elevation observed in HD patients with bacteremia, as the pathogens in this population are typically not gram-negative. Further investigations will therefore be needed to clarify the detailed etiology of ALP elevation in bacteremia among HD patients.
Limitations
Several limitations to the present study warrant mention. First, given the relatively large size and educational function of the facilities used as the study sites, our rule may not be able to be used at other facilities with different roles in the region. Future studies should conduct external validation of our rule in different settings. Second, because we used a complete dataset for the analysis, subjects with a relatively mild clinical presentation without a detailed history or laboratory test findings were excluded. However, since the measured items in this study were not particularly special and are routinely measured in cases of suspected bacteremia, we believe that such exclusion did not strongly influence the distribution of the severity and comorbidities in the study population. Third, because this was a retrospective cohort study, we cannot deny uncertainty in the data extracted from the medical records. Validation studies should be conducted with a prospective design. Fourth, our sample population was relatively small. However, the required number of outcomes was calculated to be 50 when performing multivariate logistic analyses using five explanatory variables, and we almost met this threshold. Fifth, in the present study, given that we did not evaluate the reasons blood was drawn for culture, we cannot precisely determine the nature of HD patients, particularly feverish patients, who did not undergo blood culture. However, because clinical judgment on this point is often impossible to predict, we believe this lack of consideration actually increases the generalizability, as stated in the preceding paper, which developed a clinical prediction rule for bacteremia in the general population. Sixth, cases with undetectable bacteremia (blood culture-negative) might have existed, which is considered a limitation of blood culture. A new gold standard method for diagnosing bacteremia is needed. Seventh, this CPR is not strictly a predictor because it contains “No prior ABx within 1 week”, which increases the positivity of blood culture. However, we allowed the inclusion of this criterion for the following three reasons: A) If the CPR had been created only for subjects without prior ABx within 1 week, the discrimination ability would have been inferior to that of the BAC-HD score (data not shown). B) Since participants with prior ABx within 1 week were relatively common in the present study (16.4% in this cohort), their exclusion might have reduced the generalizability. C) Given the possibility of bacteremia in participants even with prior ABx, careful observation and treatment likely strongly benefited participants. However, we must recognize the limitations of this score with respect to the low exclusion accuracy in subjects with BAC-HD score <2 who have used ABx in the past week and make any clinical judgments carefully. Finally, in the present study, we were unable to compare the direct discrimination ability between CPRs for the general population and the BAC-HD score because of a data shortage. However, unlike the CPR in the general population, the BAC-HD score does not include items that greatly vary depending on the timing of hemodialysis. Therefore, we believe that the validity of the present CPR for HD patients will likely be higher than CPRs for the general population.
Conclusion
We developed a simple clinical prediction rule for bacteremia in maintenance HD patients. We expect that using this rule will facilitate the early detection, early treatment, and improvement of prognosis of bacteremia in HD patients.
Supporting Information
(XLSX)
Acknowledgments
The authors would like to thank Yoshiro Fujita for participating as an external consensus panelist and Digital Medical Communications for the English editing. We also thank the authors belonging to JOINT-KD for their intellectual support in the management of this study. The names and affiliations of the authors of the present study belonging to JOINT-KD are Sho Sasaki (Department of Healthcare Epidemiology, Kyoto University Graduate School of Public Health, Kyoto), Takeshi Hasegawa (Office for Promoting Medical Research, Showa University, Tokyo), Hiroo Kawarazaki (Division of Nephrology, Department of Internal Medicine, Inagi Municipal Hospital, Inagi), Atsushi Nomura (Department of Immunology, Juntendo University School of Medicine, Tokyo), Daisuke Uchida (Department of Nephrology and Hypertension, Kawasaki Municipal Tama Hospital, Kawasaki), Takahiro Imaizumi (Department of Nephrology, Toyohashi Municipal Hospital, Toyohashi), Masahide Furusho (Department of Nephrology, Aso Iizuka Hospital, Iizuka), Hiroki Nishiwaki (Division of Nephrology, Department of Medicine, Showa University Fujigaoka Hospital, Yokohama), Yugo Shibagaki (Division of Nephrology and Hypertension, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki). The name and contact e-mail of the lead author for JOINT-KD are Sho Sasaki and ssasaki@fmuac.jp, respectively.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Nielsen SL, Pedersen C, Jensen TG, Gradel KO, Kolmos HJ, Lassen AT. Decreasing incidence rates of bacteremia: a 9-year population-based study. J Infect 2014;69(1):51–59. 10.1016/j.jinf.2014.01.014 [DOI] [PubMed] [Google Scholar]
- 2.Hoen B, Paul-Dauphin A, Hestin D, Kessler M. EPIBACDIAL: a multicenter prospective study of risk factors for bacteremia in chronic hemodialysis patients. J Am Soc Nephrol 1998;9(5):869–876. [DOI] [PubMed] [Google Scholar]
- 3.Dopirak M, Hill C, Oleksiw M, Dumigan D, Arvai J, English E, et al. Surveillance of hemodialysis-associated primary bloodstream infections: the experience of ten hospital-based centers. Infect Control Hosp Epidemiol 2002;23(12):721–724. 10.1086/502000 [DOI] [PubMed] [Google Scholar]
- 4.Gilad J, Eskira S, Schlaeffer F, Vorobiov M, Marcovici A, Tovbin D, et al. Surveillance of chronic haemodialysis-associated infections in southern Israel. Clin Microbiol Infect 2005;11(7):547–552. 10.1111/j.1469-0691.2005.01168.x [DOI] [PubMed] [Google Scholar]
- 5.Ishani A, Collins AJ, Herzog CA, Foley RN. Septicemia, access and cardiovascular disease in dialysis patients: the USRDS Wave 2 study. Kidney Int 2005;68(1):311–318. 10.1111/j.1523-1755.2005.00414.x [DOI] [PubMed] [Google Scholar]
- 6.Chan KE, Warren HS, Thadhani RI, Steele DJ, Hymes JL, Maddux FW, et al. Prevalence and outcomes of antimicrobial treatment for Staphylococcus aureus bacteremia in outpatients with ESRD. J Am Soc Nephrol 2012;23(9):1551–1559. 10.1681/ASN.2012010050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fysaraki M, Samonis G, Valachis A, Daphnis E, Karageorgopoulos DE, Falagas ME, et al. Incidence, clinical, microbiological features and outcome of bloodstream infections in patients undergoing hemodialysis. Int J Med Sci 2013;10(12):1632–1638. 10.7150/ijms.6710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eleftheriadis T, Liakopoulos V, Leivaditis K, Antoniadi G, Stefanidis I. Infections in hemodialysis: a concise review—Part 1: bacteremia and respiratory infections. Hippokratia 2011;15(1):12–17. [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Jaber BL. Mortality caused by sepsis in patients with end-stage renal disease compared with the general population. Kidney Int 2000;58(4):1758–1764. 10.1111/j.1523-1755.2000.00337.x [DOI] [PubMed] [Google Scholar]
- 10.Foley RN, Guo H, Snyder JJ, Gilbertson DT, Collins AJ. Septicemia in the United States dialysis population, 1991 to 1999. J Am Soc Nephrol 2004;15(4):1038–1045. [DOI] [PubMed] [Google Scholar]
- 11.Eliakim-Raz N, Bates DW, Leibovici L. Predicting bacteraemia in validated models—a systematic review. Clin Microbiol Infect 2015;21(4):295–301. 10.1016/j.cmi.2015.01.023 [DOI] [PubMed] [Google Scholar]
- 12.Ratzinger F, Dedeyan M, Rammerstorfer M, Perkmann T, Burgmann H, Makristathis A, et al. A risk prediction model for screening bacteremic patients: a cross sectional study. PLoS One 2014;9(9):e106765 10.1371/journal.pone.0106765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Werkhoven CH, Huijts SM, Postma DF, Oosterheert JJ, Bonten MJ. Predictors of Bacteraemia in Patients with Suspected Community-Acquired Pneumonia. PLoS One 2015;10(11):e0143817 10.1371/journal.pone.0143817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takeshima T, Yamamoto Y, Noguchi Y, Maki N, Gibo K, Tsugihashi Y, et al. Identifying Patients with Bacteremia in Community-Hospital Emergency Rooms: A Retrospective Cohort Study. PLoS One 2016;11(3):e0148078 10.1371/journal.pone.0148078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lafrance JP, Iqbal S, Lelorier J, Dasgupta K, Ritchie J, Ward L, et al. Vascular access-related bloodstream infections in First Nations, community and teaching Canadian dialysis units, and other centre-level predictors. Nephron Clin Pract 2010;114(3):c204–212. 10.1159/000262303 [DOI] [PubMed] [Google Scholar]
- 16.Fontanarosa PB, Kaeberlein FJ, Gerson LW, Thomson RB. Difficulty in predicting bacteremia in elderly emergency patients. Ann Emerg Med 1992;21(7):842–848. [DOI] [PubMed] [Google Scholar]
- 17.Whitelaw DA, Rayner BL, Willcox PA. Community-acquired bacteremia in the elderly: a prospective study of 121 cases. J Am Geriatr Soc 1992;40(10):996–1000. [DOI] [PubMed] [Google Scholar]
- 18.Meyers BR, Sherman E, Mendelson MH, Velasquez G, Srulevitch-Chin E, Hubbard M, et al. Bloodstream infections in the elderly. Am J Med 1989;86(4):379–384. [DOI] [PubMed] [Google Scholar]
- 19.Mylotte JM, McDermott C. Recurrent gram-negative bacteremia. Am J Med 1988;85(2):159–163. [DOI] [PubMed] [Google Scholar]
- 20.Elhanan G, Raz R, Pitlik SD, Sharir R, Konisberger H, Samra Z, et al. Bacteraemia in a community and a university hospital. J Antimicrob Chemother 1995;36(4):681–695. [DOI] [PubMed] [Google Scholar]
- 21.Khayr WF, CarMichael MJ, Dubanowich CS, Latif RH. Epidemiology of bacteremia in the geriatric population. Am J Ther 2003;10(2):127–131. [DOI] [PubMed] [Google Scholar]
- 22.Arpi M, Renneberg J, Andersen HK, Nielsen B, Larsen SO. Bacteremia at a Danish university hospital during a twenty-five-year period (1968–1992). Scand J Infect Dis 1995;27(3):245–251. [DOI] [PubMed] [Google Scholar]
- 23.Girndt M. Bacteraemia in haemodialysis patients—not always Staphylococcus aureus. Nephrol Dial Transplant 2015;30(7):1055–1057. 10.1093/ndt/gfv223 [DOI] [PubMed] [Google Scholar]
- 24.Vandecasteele SJ, Boelaert JR, De Vriese AS. Staphylococcus aureus infections in hemodialysis: what a nephrologist should know. Clin J Am Soc Nephrol 2009;4(8):1388–1400. 10.2215/CJN.01590309 [DOI] [PubMed] [Google Scholar]
- 25.Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, et al. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162(1):W1–W73. 10.7326/M14-0698 [DOI] [PubMed] [Google Scholar]
- 26.Murray EC, Marek A, Thomson PC, Coia JE. Gram-negative bacteraemia in haemodialysis. Nephrol Dial Ttransplantation 2015;30(7):1202–1208. [DOI] [PubMed] [Google Scholar]
- 27.Fram D, Taminato M, Ponzio V, Manfredi SR, Grothe C, Batista RE, et al. Risk factors for morbidity and mortality of bloodstream infection in patients undergoing hemodialysis: a nested case-control study. BMC Res Notes 2014;7:882 10.1186/1756-0500-7-882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crowley L, Wilson J, Guy R, Pitcher D, Fluck R. Chapter 12 Epidemiology of Staphylococcus aureus bacteraemia amongst patients receiving dialysis for established renal failure in England in 2009 to 2011: a joint report from the Health Protection Agency and the UK Renal Registry. Nephron Clin Pract 2012;120 Suppl 1:c233–245. [DOI] [PubMed] [Google Scholar]
- 29.Thomson PC, Stirling CM, Geddes CC, Morris ST, Mactier RA. Vascular access in haemodialysis patients: a modifiable risk factor for bacteraemia and death. QJM 2007;100(7):415–422. 10.1093/qjmed/hcm040 [DOI] [PubMed] [Google Scholar]
- 30.Hosmer DW Jr, Lemeshow S. Applied logistic regression: John Wiley & Sons; 2004. [Google Scholar]
- 31.Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, et al. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21(1):128–138. 10.1097/EDE.0b013e3181c30fb2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efron B. Bootstrap methods: another look at the jackknife: Springer; 1992. [Google Scholar]
- 33.Saxena AK, Panhotra BR, Al-Mulhim AS. Vascular access related infections in hemodialysis patients. Saudi J Kidney Dis Transpl 2005;16(1):46–71. [PubMed] [Google Scholar]
- 34.Katneni R, Hedayati SS. Central venous catheter-related bacteremia in chronic hemodialysis patients: epidemiology and evidence-based management. Nat Clin Pract Nephrol 2007;3(5):256–266. 10.1038/ncpneph0447 [DOI] [PubMed] [Google Scholar]
- 35.Pisoni RL, Arrington CJ, Albert JM, Ethier J, Kimata N, Krishnan M, et al. Facility hemodialysis vascular access use and mortality in countries participating in DOPPS: an instrumental variable analysis. Am J Kidney Dis 2009;53(3):475–491. 10.1053/j.ajkd.2008.10.043 [DOI] [PubMed] [Google Scholar]
- 36.Ethier J, Mendelssohn DC, Elder SJ, Hasegawa T, Akizawa T, Akiba T, et al. Vascular access use and outcomes: an international perspective from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplantation 2008;23(10):3219–3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tung CB, Tung CF, Yang DY, Hu WH, Hung DZ, Peng YC, et al. Extremely high levels of alkaline phosphatase in adult patients as a manifestation of bacteremia. Hepato-gastroenterology 2005;52(65):1347–1350. [PubMed] [Google Scholar]
- 38.Cohen J. The immunopathogenesis of sepsis. Nature 2002;420(6917):885–981. 10.1038/nature01326 [DOI] [PubMed] [Google Scholar]
- 39.Peters E, Heemskerk S, Masereeuw R, Pickkers P. Alkaline phosphatase: a possible treatment for sepsis-associated acute kidney injury in critically ill patients. Am J Kidney Dis 2014;63(6):1038–1048. 10.1053/j.ajkd.2013.11.027 [DOI] [PubMed] [Google Scholar]
- 40.Peters E, van Elsas A, Heemskerk S, Jonk L, van der Hoeven J, Arend J, et al. Alkaline phosphatase as a treatment of sepsis-associated acute kidney injury. J Pharmacol Exp Ther 2013;344(1):2–7. 10.1124/jpet.112.198226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.