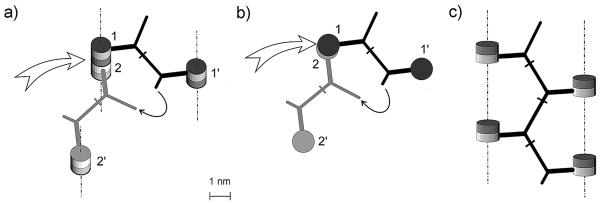
FIG. 2.
Formation of the tetrameric muropeptide from murein after virtual digestion with muramidase. Shown are considerations according to the original scaffold model (a and b) and representation of the advanced, “ladder-type” scaffold model (c). (a) Four muropeptide units belong to three strands and are located in two horizontal planes. Twin barrels correspond to the disaccharide units; those marked with a darker color (1 and 1′) are above those marked with a lighter color (2 and 2′). Vertical orientation of the strands is indicated by dashed-and-dotted lines. The white arrow points to the enzyme-cleavable bond between disaccharide units. The semicircular arrow points to the ends of short and long peptide arms which belong to two bridges that need to be connected. (b) Topside view of molecular geometry (topology) shown in panel a. Barrels filled with a darker color (1 and 1′) are located in a plane that is above the plane of a paper; lighter barrels (2 and 2′) are in the paper plane. (c) Unstressed ladder-like conformation of the tetrameric muropeptide with a zigzag geometry of the major peptide chain and four disaccharide substituents located in one plane. The atomic parameters of the muropeptides in question are identical to those presented in Fig. 1.