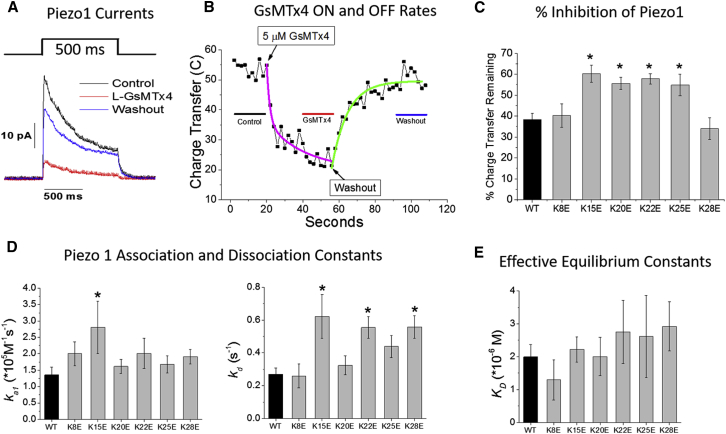
Figure 2.
(A) Typical averaged currents in an outside-out patch from a cell expressing Piezo1, before (black), during (red), and after (blue) application of 5 μM WT GsMTx4. Patch currents were recorded at −50 mV pipette potential. (B) A typical experiment (not the same as A) shows the integrated current (charge transfer) levels diminishing over time after GsMTx4 application and recovering after washout. The regions from which the charge transfer levels were determined are indicated by lines in (B) with colors coordinated to the traces in (A). The mean reduction of Piezo 1 charge transfer by the different peptides is shown in (C). The average charge transfer from the 500 ms steps before peptide application (control line in B) were set to 100% to normalize the inhibition of different patches. The percent values in (C) represent the average fractional reduction in steady-state charge-transfer determined from the region indicated by the red line in (B). For a summary of individual patch data, see Fig. S4. Asterisks represent significantly different means at α = 0.05. Time constants were determined by modeling the decay and recovery phases with exponential fits (magenta and green curves, respectively, in B). The net association (ka) and dissociation (kd) rate constants for inhibition of Piezo1 (D) were calculated from the first-order time constants derived from the exponential fits to the data (mean constant values ± SE). The number of patches analyzed to compute the mean kas was WT = 38, K8E = 15, K15E = 14, K20E = 19, K22E = 16, K25E = 15, and K28E = 21; and for kds the numbers were WT = 28, K8E = 17, K15E = 16, K20E = 15, K22E = 18, K25E = 14, and K28E = 19. Control WT GsMTx4 constants are shown in black, analogs in gray. (E) The calculated effective equilibrium constants (KD) ± SE are shown. Analogs that were significantly different (α = 0.05) from WT are denoted by an ∗. To see this figure in color, go online.