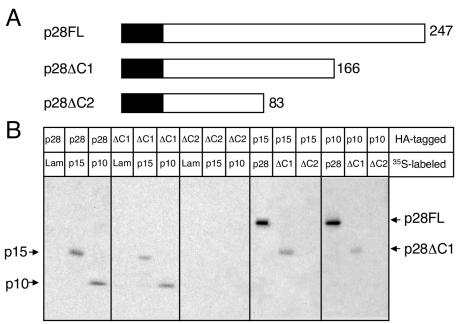
FIG. 6.
The carboxy terminus of p28 is not required for interactions with p10 and p15. (A) p28 deletion mutagenesis. A schematic of full-length 247-amino-acid p28 (p28FL) is shown with the two carboxy-terminal truncation mutants, p28ΔC1 and p28ΔC2, below p28FL. The number of p28 amino acid residues remaining is indicated to the right of each protein. The c-Myc or HA epitope tag is represented as a black box fused to the amino terminus of each p28 protein and is not drawn to scale. (B) Coimmunoprecipitation of in vitro-expressed proteins. The proteins were translated, using reticulocyte lysates, as fusions to c-Myc epitope tags in the presence of [35S]methionine or as fusions to HA epitope tags in the absence of radiolabel. Equal amounts of radiolabeled and nonradiolabeled lysates were combined, and the proteins were immunoprecipitated using rabbit polyclonal α-HA antiserum. Interacting 35S-labeled proteins were resolved in an SDS-12% polyacrylamide gel and analyzed by fluorography. The identities of coprecipitating proteins are shown on the right of the gel.