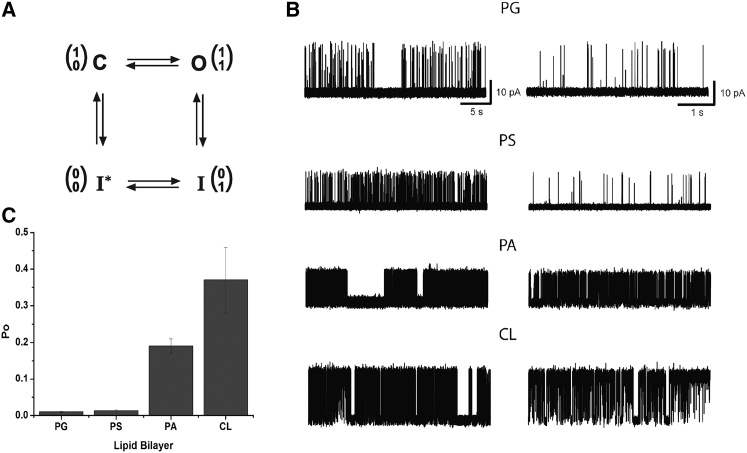
Figure 1.
(A) Four-state minimal gating model with upper (inactivation) and lower (activation) gates in the conduction pathway of the KcsA channel. Gate positions (0 for closed and 1 for opened) are diagrammed in one-column matrices, with the upper gate in the top row and the lower gate in the bottom row. C, closed channel in resting state; O, open channel; I and I∗, inactivated states as in Ref. (22). (B) Representative current traces of KcsA recorded in different anionic phospholipid environments. The specific anionic phospholipid is indicated above the current traces. On the left, long 30 s traces are presented; on the right, 5 s traces are shown. All experiments were performed at +100 mV in symmetrical 150 mM KCl solution at pH 7.0 on the cis side and pH 4.0 on the trans side. Lipid bilayers were composed of 70% anionic phospholipid and 30% neutral DOPC. (C) Open probability (PO) of KcsA in different anionic phospholipids. All experiments were performed in symmetrical 150 mM KCl solution at pH 7.0 on the cis side and pH 4.0 on the trans side. Lipid bilayers were composed of 70% anionic lipid and 30% neutral DOPC. Bottom: overall PO values calculated for entire recordings of the single-channel currents conducted by KcsA, including long closed periods, for different anionic phospholipid environments (see main text). PO values are given in the text as mean ± SEM.