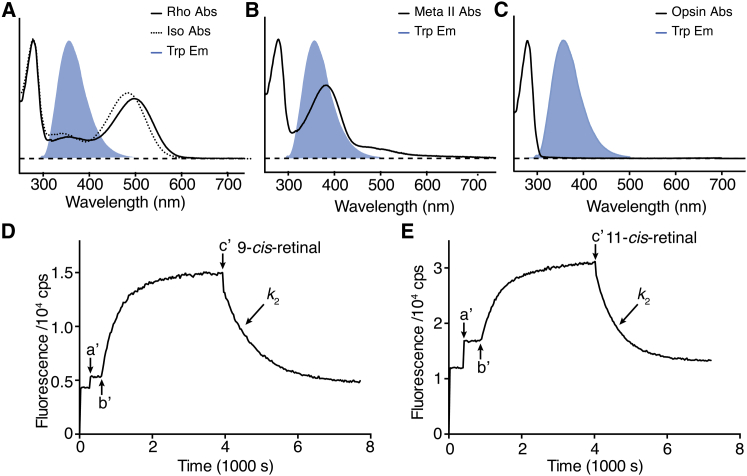
Figure 2.
Measurement of the binding kinetics between opsin and retinals. The absorption spectra of Rho and isoRho (A), Meta-II state (B), and opsin (C) are overlaid with the fluorescence emission peaks of Trp (blue, maximum at 330 nm). The spectra are normalized to the intensities of the absorption and fluorescence emission peaks. (D and E) Tryptophan fluorescence time course traces are presented. The dark-state pigment was added into the assay buffer at arrow a′. The sample was then photobleached at arrow b′ to form the Meta-II state. After Meta-II decay was close to completion at arrow c′, ligand 11CR was added to initiate pigment regeneration. The binding between opsin and 9CR (k2 = (0.67 ± 0.03) × 103m−1 s−1) was slightly slower than that between opsin and 11CR (k2 = (1.08 ± 0.04) × 103m−1 s−1). To see this figure in color, go online.