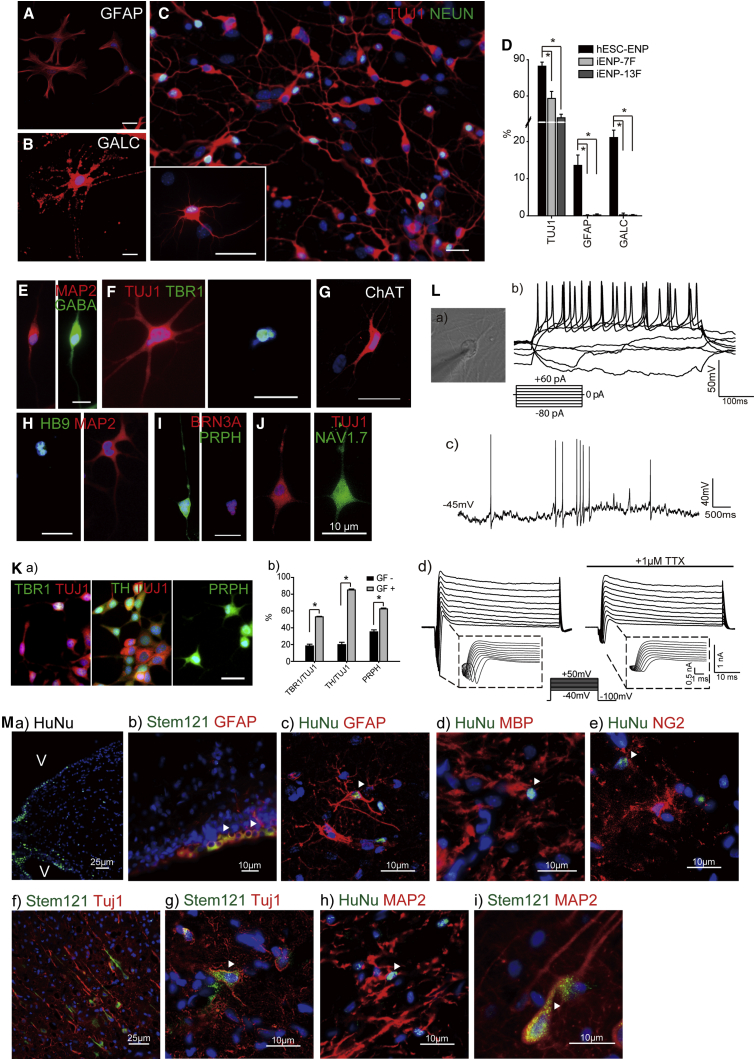
Figure 5.
Multipotency of iENP-7F In Vitro and In Vivo
(A–C) ICC staining of differentiated iENP-7F using antibodies against the glial marker GFAP (A), oligodendrocyte marker GALC (B), and neuronal markers, as indicated (C).
(D) Quantification and comparison of TUJ1+, GFAP+, and GALC+ cells in differentiated hESC-ENPs, iENP-7F, and iENP-13F.
(E–J) ICC staining of differentiated iENP-7F with antibodies against CNS and PNS neuronal antigens, as indicated.
(K) Lineage-specific cues promote the generation of specific neuronal subtypes from iENP-7F. (a) ICC characterization of differentiated iENP-7F under neuronal subtype-specific differentiation conditions using antibodies against CNS and PNS neuronal antigens, as indicated. (b) Quantification of iENP-7F-derived neuronal subtypes induced by the conditions described in Figure 4Na. GF−, without inducers; GF+, with inducers.
(L) Whole-cell patch-clamp recordings of iENP-7F-derived neurons. (a) Current recording from a neuron at 4–6 weeks. (b) Action potentials were induced by current steps from −80 to +60 pA. (c) Spontaneously firing action potentials were recorded at a subthreshold oscillatory potential of −40 mV. (d) Inward Na+ currents and outward Ca2+ currents were induced by voltage steps from −40 to +50 mV. The inward Na+ currents could be blocked by tetrodotoxin (TTX).
(M) In vivo transplantation of iENP-7F. (a) IHC staining of the corpus callosum containing iENP-7F transplants using an antibody against human nuclear antigen (HuNu), revealing migration of iENPs into ventricular zones. (b–i) IHC analysis of brain cryosections at 12 weeks post-transplantation using antibodies against HuNu or Stem121 and the indicated neural antigens. Arrowheads indicate the cells expressing human-specific markers and neural markers.
All quantitative data were obtained from three independent experiments and are presented as means ± SD. ∗p < 0.05. (A–I and K) Scale bar, 10 μm. See also Figure S3.