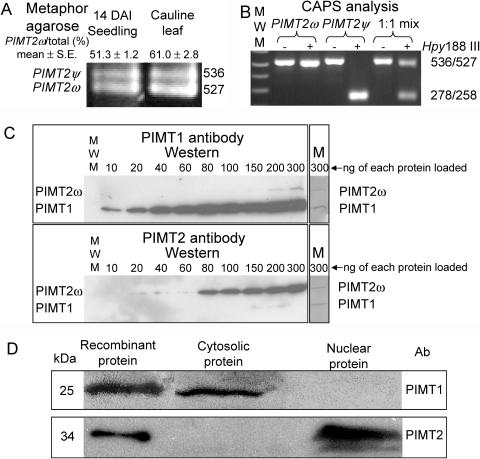
Figure 2.
A, Amplicons from RT-PCR reactions from DNA-free, seedling, and leaf RNA produced using primers CH5F-0 and CH5R-0 (Supplemental Table I) were separated in a 2% MetaPhor gel, stained using SYBR-Gold, and photographed. Two bands were discernable of a molecular mass consistent with an alternative splicing event that retained (PIMT2ψ) or eliminated (PIMT2ω) the 9 3′-most nt of the first intron. The average ± se of multiple estimates of PIMT2ω abundance relative to total PIMT2 transcript based on CAPS, metaphor agarose, or polyacrylamide separation are provided. B, Inclusion of an extra 9 nt in the larger of the two transcripts through alternative 3′ splice site selection results in a CAPS using Hpy188 III. The 536-bp PIMT2ψ amplicon is cleaved into two smaller, comigrating products of 278 and 258 bp that are discernable from the larger 527-bp PIMT2ω amplicon on a 1% w/v agarose gel. −, No restriction endonuclease was added. +, Hpy188 III was added to the amplicon. PIMT2ω, Amplicon from plasmid containing PIMT2ω insert; PIMT2ψ, amplicon from plasmid containing PIMT2ψ insert; 1:1, amplicon generated from an equal mixture of plasmid containing PIMT2ψ or PIMT2ω. C, Polyclonal antibodies to PIMT1 and PIMT2 were produced and affinity purified. Recombinant PIMT1 and PIMT2 proteins produced from pET23d with and without a carboxy-terminal hexahistidyl tag (HIS-tag), respectively, were mixed in equal proportions and loaded in increasing amounts (from 10–300 ng isozyme−1 well−1) in two 12% SDS-polyacrylamide gels. Following electrophoresis and transfer to membrane, representative blots were challenged with either PIMT1 or PIMT2 antibody. After chemiluminescent detection, the membranes (M) were stained with Coomassie Blue to exhibit recombinant protein amounts. D, Western blots of the cytosolic and nuclear fractions from 24-h imbibed seeds challenged with affinity-purified, polyclonal antibodies to PIMT1 or PIMT2 protein. Recombinant protein, produced from pET23d with (PIMT1) or without (PIMT2) a carboxy-terminal HIS-tag, were run as controls.