Abstract
Background
Hepatic steatosis is increasing worldwide. Whether HIV and its associated metabolic perturbations exacerbate steatosis is unclear. Sex differences in adipose tissue distribution may also affect steatosis risk. We examined the contribution of HIV and sex to steatosis.
Methods
Using magnetic resonance imaging and spectroscopy, visceral adipose tissue (VAT) and liver fat fraction (LFF) were measured in 121 HIV-infected and 107 uninfected men and women without viral hepatitis. Differences in LFF by HIV status and sex were evaluated using multivariable linear regression, adjusting for demographic, lifestyle, VAT, homeostasis model assessment-estimated insulin resistance (HOMA-IR), and HIV-related factors.
Results
HIV-infected women had lower LFF than uninfected women (demographic-adjusted mean:1.9% vs. 3.1%;p=0.028); LFF was similar in HIV-infected and uninfected men (4.6% vs. 4.1%;p=0.78). HIV-infected and uninfected women had less VAT than men (median:139cm3 and 161cm3 vs 201cm3 and 188cm3, respectively). After adjustment, HIV-infected women had 34%(95%CI:−54%,−5.5%) lower LFF than uninfected women, whereas there was little difference in men (−5.5%;95%CI:−26%,21%). Among HIV-infected persons, greater VAT and HOMA-IR were associated with greater LFF. HIV-related factors (CD4 count, HIVRNA level, or antiretroviral therapy use) had little association with LFF. Although HIV-infected men had 81%(95%CI:32%,148%) greater LFF than HIV-infected women, the association was attenuated after multivariable adjustment (25%;95%CI:−9.1%,73%).
Conclusion
Contrary to expectation, HIV infection is not associated with greater steatosis compared to uninfected adults. It is possible that less fat is stored in the liver to maintain subcutaneous fat (which is reduced in HIV) and the effect is magnified in HIV-infected women, who also have less VAT.
Keywords: HIV, Hepatic Steatosis, Non-Alcoholic Fatty Liver Disease, Sex Differences
Introduction
The advent of effective antiretroviral therapy (ART) has led to dramatic improvements in the life expectancy of HIV-infected persons; yet, HIV-infected persons remain at greater risk of death than HIV-uninfected persons due to the emergence of non-AIDS related morbidity that may be accelerated in the setting of HIV infection[1]. Chronic liver disease is now a leading cause of non-AIDS-related death, representing 13% of all deaths in this patient population. While coinfection with viral hepatitis is the primary cause of liver disease, hepatic steatosis is emerging as a major contributor[2–4].
Steatosis, which is part of the spectrum of non-alcoholic fatty liver disease (NAFLD), is increasing in prevalence[5] and is reported to range from 10 – 46%[6–8] in the general population, paralleling the rise in obesity and associated conditions including insulin resistance and dyslipidemia[9]. NAFLD can progress to cirrhosis and its complications, including hepatocellular carcinoma in up to 20% of patients[10]. Steatosis is also reported in 14 to 54% of HIV-monoinfected adults in the modern era of ART[2–4, 11–15]. HIV-infected adults may be at particular risk because HIV-related perturbations in adipose tissue and insulin resistance are common[3, 4, 16]. However, few have examined the prevalence and factors associated with steatosis in HIV-monoinfected relative to uninfected persons.
Less is known about sex differences in the pathogenesis of steatosis in the setting of HIV infection. Studies evaluating the influence of sex on NAFLD in the general population have been conflicting[8, 17–22]. Two studies showed that women had a higher prevalence than men[20, 21]. In one, steatosis was defined by an elevated ALT in the absence of alcohol use.[21] The other found a greater risk of biopsy-proven non-alcoholic steatohepatitis (NASH) in women with NAFLD compared to men[20]. By contrast, a study in NHANES found that men were more than two times as likely as women to have steatosis (defined by an elevated ALT in the absence of alcohol use)[19]. The Dallas Heart Study found that White men had a two-fold higher prevalence of steatosis measured by magnetic resonance spectroscopy (MRS) than White women, whereas sex differences were not observed in Blacks and Hispanics[17]. A limitation in interpreting these findings is the different modalities used to measure NAFLD; liver biopsy is generally considered the clinical gold standard and MRS the non-invasive gold standard to measure steatosis. Furthermore, it is unclear if women were pre- or postmenopausal in these studies; some studies have shown that the protective effect of estrogen on steatosis is lost following the menopausal transition[23–25].
We evaluated the contributions of HIV and sex to steatosis, after adjustment for demographic, lifestyle, body composition and metabolic factors using data collected from two ethnically diverse cohorts of HIV-infected and uninfected adults. Visceral adipose tissue (VAT) volume was measured using magnetic resonance imaging (MRI) and liver fat fraction was calculated from MRS.
Methods
Study Population
The Women’s Interagency HIV Study (WIHS) is a multicenter prospective cohort study that enrolled a total of 4,982 women (3,678 HIV-infected and 1,304 HIV-uninfected) between 1994 and 2015 from eleven U.S. cities. Baseline socio-demographic characteristics and HIV risk factors were similar between HIV-infected and uninfected women[26, 27]. An institutional review board approved study protocols and consent forms, and each participant gave written informed consent. Every six months, participants complete a comprehensive physical examination, provide biological specimens, and complete an interviewer-administered questionnaire.
From December 2003 through July 2015, 139 women from the San Francisco (SF) WIHS site with HIV monoinfection (n=58), HIV/HCV coinfection (n=47), HCV monoinfection (n=5), and neither HIV nor HCV infection (n=29) were enrolled into a Steatosis substudy to investigate the contribution of HIV, HCV, and metabolic factors to hepatic steatosis. From October 2010 through June 2014, 224 participants (98% men) between the ages of 35 and 70 with HIV monoinfection (n=64), HIV/HCV coinfection (n=27), HCV monoinfection (n=55), and neither HIV nor HCV infection (n=78) were enrolled into the Study of Visceral Adiposity, HIV, and HCV: Biologic Mediators of Hepatic Steatosis from the SF VA Medical Center through posted flyers, patient to patient referrals, and providers approaching patients in clinic.
In both studies, patients with evidence of hepatitis B surface antigenemia, prior HCV treatment, history of decompensated cirrhosis, metal in their body, or a wide abdominal girth that would not allow them to fit into the MRI scanner were excluded from enrollment. For our analysis, HCV-infected participants from both studies were excluded in order to examine the relationship of HIV and sex with steatosis in the absence of viral hepatitis infection.
Ascertainment of MRS-measured liver fat fraction
MRS was performed on a 1.5 Tesla (T) whole body clinical scanner (General Electric Healthcare, Waukesha, WI) from December 2003 to February 2010 and on a 3T whole body scanner (General Electric Healthcare, Waukesha, WI) beginning in March 2010. Data collected from the 1.5T scanner were standardized to values collected from the 3T scanner. All VAHH participants were scanned using the same 3T scanner as used in WIHS participants. A time series of 128 (1.5T) or 64 (3T) acquisitions of spectra were obtained from an 8 cubic centimeter (cm3), single voxel region of tissue and were analyzed with motion correction and T2 relaxation time correction algorithms using a standardized MRS protocol[28]. The peak areas under the resonance frequencies of lipids and unsuppressed water were calculated for each participant and the liver fat fraction (LFF) derived as the ratio of the total lipids measure to the total lipids plus unsuppressed water measure. The LFF was then multiplied by 100 and expressed as a percent. A mean inter-examination coefficient of variation for MRS-measured LFF of 11.9% (range 2.8 – 20.3%) in a sample of 9 controls confirmed its reproducibility. Steatosis was defined as a LFF≥5%.
Ascertainment of Body Composition and Metabolic factors
VAT and abdominal subcutaneous adipose tissue (SAT) were calculated as the mean volume (cm3) of the visceral and subcutaneous compartments, respectively by MRI of the L2/3, L3/4, and L4/5 intervertebral disk levels. Body mass index (BMI) was calculated from height and weight in kilogram per meter squared (kg/m2). Leg fat mass (kg) was determined from dual energy X-ray absorptiometry (DXA) scan (Lunar Prodigy, Madison, Wisconsin). The Homeostasis Model Assessment estimated insulin resistance (HOMA-IR) [fasting insulin (μU/mL) × glucose (mg/dL)/405].
Covariates
Our primary predictors were HIV infection status (defined by prior documentation of a positive HIV EIA confirmed by Western blot) and sex. Candidate covariates included: sociodemographic factors (age, sex, race/ethnicity), menopausal status categorized by Anti-Mullerian Hormone (AMH) level into premenopause (AMH>0.9ng/ml), perimenopause (AMH<0.9ng/ml within the past 5 years), and postmenopause (AMH<0.9ng/ml for more than 5 years), lifestyle factors [history of injection drug use, alcohol use (none; >0–7 drinks/week; 7–12 drinks/week; >12 drinks/wk); marijuana use (current vs. not current), smoking (current vs. not current)], body composition (waist circumference, waist-to-hip ratio, body mass index, MRI-measured VAT volume, DXA-measured leg fat amount), metabolic factors [diabetes mellitus diagnosis (defined by a confirmed elevated fasting glucose, elevated hemoglobin A1C, or self-report of anti-diabetes medications) and insulin resistance estimated using the homeostasis model assessment (HOMA-IR)], and liver-related factors [aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, albumin, and AST to platelet ratio index (APRI), a serum marker of fibrosis]. HIV-related factors included current CD4, nadir CD4, current HIV RNA, history of clinical AIDS, and current use of highly active antiretroviral therapy (HAART) and ART by class. Because VAT and SAT were missing in 14% of participants, we used the Markov chain Monte Carlo method to impute missing covariates.
Statistical analysis
We compared sociodemographic and clinical characteristics in women and men stratified by HIV status using the t-test or Kruskal-Wallis test for continuous variables and chi-square test or Fisher’s exact test for categorical variables where appropriate.
We used multivariable linear regression with robust standard errors to evaluate the association of HIV and sex with LFF after adjustment for lifestyle, body composition and metabolic factors. LFF was found to be right skewed and was therefore log-transformed for analysis; results were back-transformed to produce estimated percentage differences. We also used logistic regression to investigate the association of the same covariates with the presence of steatosis.
To determine whether HIV was independently associated with the LFF in women and men, we created multivariable models which were initially stratified by sex, and then pooled women and men and tested for HIV by sex interactions. We sequentially adjusted for sociodemographic factors and then lifestyle, body composition and metabolic factors. We next constructed separate models stratified by HIV status in order to identify correlates of LFF within each group. A stepwise regression with p-value of 0.10 or less was used for entry and retention; the candidate variables were selected based upon their hypothesized associations with steatosis. Age and race/ethnicity were retained in all models.
All analyses were performed using the SAS system, version 9.4 (SAS Institute Inc., Cary, NC).
Results
Cohort Characteristics
Table 1 shows the demographic and clinical characteristics of the 87 women and 142 men by HIV status. On average, women were younger than men, and uninfected women were the youngest. Women were more likely than men to be of African-American race and Hispanic ethnicity and to report abstaining from alcohol. A greater proportion of HIV-infected women were postmenopausal compared to uninfected women. HIV-infected men and women also appeared more likely to report not currently smoking than HIV-uninfected men and women. All body composition parameters appeared lower in the HIV-infected men and women compared to the respective uninfected men and women, except for VAT in men. HIV-infected women had lower HOMA-IR than uninfected women, whereas HIV-infected men had greater HOMA-IR than uninfected men, though these associations did not reach statistical significance. Among the HIV-infected participants, men were more likely than women to have an undetectable HIV RNA level, to be on HAART, and have a history of clinical AIDS; both men and women had a median CD4 count >500cells/ul.
Table 1.
Demographic and Clinical Characteristics of Women and Men by HIV Status
| Median (IQR) or % | Women
|
Men
|
||||
|---|---|---|---|---|---|---|
| HIV-infected (n=58) | HIV-uninfected (n=29) | p-value | HIV-infected (n=64) | HIV-uninfected (n=78) | p-value | |
| Demographics | ||||||
| Age | 50 (47, 55) | 40 (35, 53) | 0.004 | 53 (47, 61) | 53 (47, 57) | 0.12 |
| Race: | ||||||
| AA | 52% | 62% | 0.62 | 20% | 53% | 0.0004 |
| White | 28% | 24% | 77% | 45% | ||
| Other | 21% | 14% | 3.1% | 2.6% | ||
| Hispanic | 60% | 69% | 0.43 | 20% | 14% | 0.33 |
| Postmenopause | 32% | 21% | 0.04 | - | - | |
| Lifestyle | ||||||
| Alcohol: | ||||||
| none | 45% | 52% | 0.17 | 23% | 33% | 0.007 |
| >0–7 drinks/wk | 41% | 28% | 61% | 33% | ||
| >7–12 drinks/wk | 8.6% | 3.4% | 6.3% | 7.7% | ||
| >12 drinks/wk | 5.2% | 17% | 9.4% | 26% | ||
| Current smoker | 33% | 48% | 0.16 | 27% | 42% | 0.09 |
| Current MJ use | 36% | 45% | 0.44 | 30% | 26% | 0.59 |
| IDU ever | 6.9% | 14% | 0.29 | 19% | 10% | 0.15 |
| Metabolic | ||||||
| BMI (kg/m2) | 27 (24, 32) | 32 (27, 35) | 0.05 | 26 (23, 29) | 27 (25, 31) | 0.03 |
| Waist Circ (cm) | 94 (87, 102) | 103 (86, 112) | 0.23 | 93 (84, 103) | 98 (89, 110) | 0.02 |
| VAT (cm2) | 139 (101, 180) | 161 (97, 208) | 0.86 | 201 (136, 275) | 188 (114, 234) | 0.08 |
| Abd SAT (cm2) | 298 (220, 412) | 441 (229, 542) | 0.18 | 162 (106, 264) | 218 (141, 327) | 0.12 |
| Leg fat (kg) | 9.5 (7.7, 12.2) | 12.6 (9.3, 16.5) | 0.17 | 4.3 (2.6, 7.1) | 6.7 (4.3, 8.6) | 0.001 |
| HOMA-IR | 1.66 (0.76, 2.57) | 2.23 (0.97, 3.44) | 0.25 | 2.02 (1.06, 4.03) | 1.48 (0.95, 2.20) | 0.08 |
| Liver-related | ||||||
| ALT (U/L) | 15 (12, 20) | 16 (14, 22) | 0.64 | 26 (21, 34) | 20 (16, 25) | 0.001 |
| AST (U/L) | 18 (16, 25) | 21 (15, 23) | 0.68 | 24 (20, 31) | 21 (18, 25) | 0.01 |
| Albumin (g/dL) | 4.3 (4.1, 4.5) | 4.2 (4.0, 4.4) | 0.33 | 4.5 (4.3, 4.7) | 4.4 (4.2, 4.6) | 0.39 |
| APRI | 0.23 (0.19, 0.29) | 0.19 (0.14, 0.27) | 0.10 | 0.39 (0.30, 0.52) | 0.35 (0.25, 0.44) | 0.13 |
| HIV-related | ||||||
| HIV RNA (copies/mL) | 48 (20, 88) | - | 40 (24, 48) | - | . | |
| HIV RNA undetectable | 60% | - | 86% | - | . | |
| CD4 (/mm3) | 569 (440, 810) | - | 610 (395, 795) | - | . | |
| CD4 nadir | 278 (134, 381) | - | 200 (71, 385) | - | . | |
| ART exposure (yr) | 5.2 (2.9, 11.7) | - | 8.8 (5.7, 15.0) | - | ||
| Current HAART | 73% | - | 97% | - | . | |
| Clinical AIDS | 33% | - | 41% | .- | . | |
AA=African American; wk=week; MJ=marijuana; IDU=injection drug use; BMI=body mass index; Circ=circumference; Abd SAT=abdominal subcutaneous adipose tissue; HOMA-IR=homeostasis model assessment of insulin resistance; ALT=alanine aminotransferase; AST=aspartate aminotransferase; APRI=AST Platelet Ratio Index; ART=Antiretroviral Therapy; HAART=highly active antiretroviral therapy; AIDS=Acquired Immunodeficiency Syndrome
Association of HIV with liver fat fraction in men and women
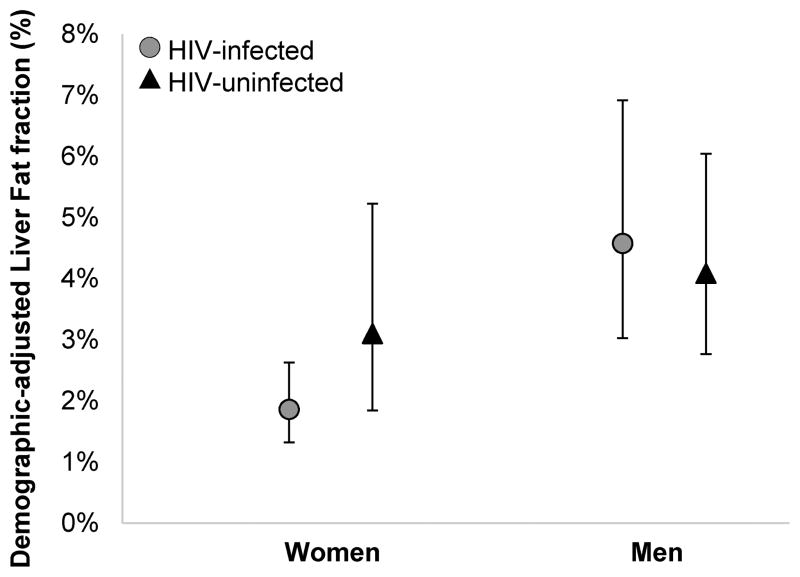
We first compared LFF in HIV-infected and uninfected participants, stratified by sex, with adjustment for age and race/ethnicity (Figure 1). HIV-infected women had a lower LFF relative to uninfected women (marginal adjusted mean: 1.9% vs. 3.1%;p=0.028). By contrast, LFF was similar in HIV-infected and uninfected men (4.6% vs. 4.1%;p=0.78).
Figure 1.
Age and race/ethnicity-adjusted mean liver fat fraction (expressed in percent) in HIV-infected and uninfected women and men.
We next performed multivariable adjustment to determine whether HIV infection was independently associated with differences in the LFF in men and women (Table 2a). After controlling for demographic factors, HIV-infected women had 35% (95% CI:−56%,−4.5%) lower LFF than uninfected women. This difference remained statistically significant and changed little after adjustment for demographic, lifestyle and metabolic factors, and after additional adjustment for liver fibrosis severity using APRI. By contrast, there was little difference in the LFF between HIV-infected and uninfected men in demographic-adjusted (+5.1%;p=0.78) or fully adjusted analysis (−5.3%;p=0.66). However, the HIV-by-sex interaction on LFF did not reach statistical significance (p=0.10 in the fully adjusted model).
Table 2a.
Association of HIV infection with MRS-measured liver fat fraction in men and women
| HIV-infected vs. uninfected control | Women
|
Men
|
p-value for HIV-gender interaction | ||
|---|---|---|---|---|---|
| % effect (95% CI) | p-value | % effect (95% CI) | p-value | ||
| Adjusted for demographics | −35% (−56%, −4.5%) | 0.028 | 5.1% (−26%, 49%) | 0.78 | 0.13 |
| Adjusted for demographics, lifestyle, & metabolic factors | −34% (−54%, −5.5%) | 0.023 | −5.5% (−26%, 21%) | 0.66 | 0.14 |
| Adjusted for demographics, lifestyle, metabolic factors & APRI | −36% (−55%, −7.9%) | 0.016 | −5.3% (−26%, 21%) | 0.66 | 0.10 |
MRS=magnetic resonance spectroscopy; CI=confidence interval; APRI=AST (Aspartate Aminotransferase) Platelet Ratio Index
All models used multiple imputation of 20 sets for missing data except demographics where there are no missing values
Our results were similar when we examined the association of HIV with the presence of steatosis. Steatosis was present in 17% of the HIV-infected women, 33% of the uninfected women, 41% of the HIV-infected men, and 33% of the uninfected men. After adjustment for age and race/ethnicity, HIV-infected women had 82% lower odds of having steatosis compared to uninfected women (Table 2b). This difference persisted after adjustment for demographic, lifestyle and metabolic factors, and additional adjustment for liver fibrosis severity. By contrast, HIV-infected men had 20% higher odds of having steatosis compared with uninfected men in demographic-adjusted analysis, although the association was not statistically significant. We did observe a significant HIV-by-sex interaction after demographic adjustment (p=0.019), but not after adjustment for demographic, lifestyle, and metabolic factors (p=0.162) and additional adjustment for liver fibrosis severity (p=0.119).
Table 2b.
Association of HIV infection with presence of steatosis* in men and women
| HIV-infected vs. uninfected control | Women
|
Men
|
p-value for HIV-gender interaction | ||
|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | ||
| Adjusted for demographics | 0.18 (0.05, 0.62) | 0.006 | 1.20 (0.56, 2.58) | 0.633 | 0.019 |
| Adjusted for demographics, lifestyle, & metabolic factors | 0.21 (0.05, 0.91) | 0.037 | 0.80 (0.25, 2.57) | 0.711 | 0.162 |
| Adjusted for demographics, lifestyle, metabolic factors & APRI | 0.17 (0.04, 0.81) | 0.026 | 0.75 (0.23, 2.43) | 0.629 | 0.119 |
Steatosis defined as liver fat fraction ≥5%; OR=odds ratio; CI=confidence interval; APRI=AST (Aspartate Aminotransferase) Platelet Ratio Index
All models used multiple imputation of 20 sets for missing data except demographics where there are no missing values
Factors associated with liver fat fraction in the HIV-infected and in the uninfected groups
We next sought to identify factors associated with LFF in HIV-infected and uninfected persons (Table 3). Among the HIV-infected, factors associated with greater LFF in unadjusted analysis included male gender, greater VAT, greater HOMA-IR, higher ALT and higher serum albumin, while African-American race was associated with lower LFF. HIV-related factors including CD4 count (−9.8% per doubling of CD4; 95%CI: −26%, 9.4%), HIVRNA (−3.3% per doubling; 95%CI:−9.4%, 3.2%), or antiretroviral therapy use (58.1%; 95%CI:−7.8%, 171.3%) had little association with LFF in unadjusted analysis and were therefore not included in the final model. After multivariable adjustment, greater VAT, greater HOMA-IR and higher albumin remained independently associated with greater LFF, while the effect of male gender was attenuated (from 81% to 25%) and was no longer statistically significant.
Table 3.
Factors Associated with MRS-measured liver fat fraction in HIV-infected and uninfected men and women
| HIV-infected
|
HIV-uninfected
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |||||
|
|
|
|||||||
| % estimate (95% CI) | p-value | % estimate (95% CI) | p-value | % estimate (95% CI) | p-value | % estimate (95% CI) | p-value | |
|
|
|
|||||||
| Demographics | ||||||||
|
| ||||||||
| Age (per decade) | 17% (−2.8%, 40%) | 0.098 | −10% (−22%, 4.4%) | 0.165 | 3.6% (−16%, 28%) | 0.744 | −13% (−29%, 6.9%) | 0.185 |
| Male | 81% (32%, 148%) | <.001 | 25% (−9.1%, 73%) | 0.168 | 11% (−27%, 68%) | 0.633 | −8.0% (−46%, 58%) | 0.761 |
| Ethnicity (vs. White) | ||||||||
| Black | −47% (−62%,−26%) | <.001 | −21% (−42%, 8.4%) | 0.146 | −5.7% (−37%, 41%) | 0.775 | 13% (−21%, 61%) | 0.495 |
| Other | −6.0% (−43%, 54%) | 0.807 | 8.6% (−29%, 65%) | 0.701 | 32% (−43%, 208%) | 0.517 | −12% (−59%, 90%) | 0.753 |
| Hispanic (vs. non-Hispanic) | −20% (−44%, 13%) | 0.205 | 22% (−9.3%, 63%) | 0.191 | 27% (−16%, 91%) | 0.252 | 35% (−12%, 107%) | 0.166 |
|
| ||||||||
| Metabolic factors | ||||||||
|
| ||||||||
| VAT (per doubling) | 96% (69%, 128%) | <.001 | 58% (29%, 93%) | <.001 | 74% (48%, 104%) | <.001 | 60% (33%, 92%) | <.001 |
| HOMA-IR (per doubling) | 50% (35%, 67%) | <.001 | 18% (3.6%, 33%) | 0.012 | 43% (24%, 66%) | <.001 | 22% (6.8%, 40%) | 0.004 |
|
| ||||||||
| Liver-related factors | ||||||||
|
| ||||||||
| ALT (per doubling) | 46% (21%, 75%) | <.001 | 11% (−7.5%, 32%) | 0.27 | 56% (20%, 101%) | 0.001 | 31% (2.6%, 68%) | 0.030 |
| Albumin (per 0.1 g/dL increase) | 11% (5.5%, 16%) | <.001 | 6.4% (2.3%, 11%) | 0.002 | 3.0% (−3.3%, 9.6%) | 0.359 | 5.3% (−0.1%, 11%) | 0.056 |
VAT=Visceral adipose tissue; HOMA-IR=homeostasis model assessment of insulin resistance; ALT=alanine aminotransferase; Multiple imputations were done on models with VAT and HOMA-IR
Among the uninfected, factors associated with greater LFF in both unadjusted and adjusted analysis included greater VAT, greater HOMA-IR, and higher ALT. There was little difference in the LFF between uninfected men and women. Higher serum albumin was also associated with greater LFF after multivariable adjustment, although the association did not reach statistical significance.
Sensitivity analysis including only participants with complete data was also performed and all inferences remained the same. Among the HIV-infected, greater VAT (51%; 95%CI: 24%, 83%), greater HOMA-IR (18%; 95%CI: 4.6%, 34%) and higher albumin (6.6%; 95%CI: 2.5%, 11%) remained independently associated with greater LFF. Among the HIV-uninfected, greater VAT (62%; 95%CI: 37%, 92%), greater HOMA-IR (23%; 95%CI: 7.5%, 40%), and higher ALT (31%; 95%CI: 3.3%, 66%) remained independently associated. Similarly, higher albumin was also associated with greater LFF, but the association did not reach significance (5.4%; 95%CI: 0.2%, 11%).
Discussion
In our study of HIV-infected and uninfected adults, contrary to expectation, we found that HIV is not associated with more liver fat; instead, we found that HIV-infected women have less liver fat than uninfected women and there was little difference in the LFF between HIV-infected and uninfected men. Our findings highlight the importance of having an uninfected comparison group. Our study is also novel in that to our knowledge, no study has examined the association of HIV monoinfection with steatosis in women and none have examined sex differences in the association of HIV with steatosis. In the HIV-infected group, we found that men had an 81% greater LFF than women, but after adjustment for demographic, lifestyle, metabolic, and HIV-related factors, the association was attenuated but remained 25% greater than women. By contrast, there was little difference between men and women in the uninfected group. These findings suggest that HIV infection contributes to a sex difference in steatosis risk beyond CD4 immunosuppression, HIV viral replication, and antiretroviral exposure, which we adjusted for in our analysis. As expected, visceral adiposity and insulin resistance were strongly associated with greater liver fat in both groups.
We observed a high steatosis prevalence in HIV-infected men and women of 41% and 17%, respectively which is consistent with published reports in HIV-monoinfected adults[2–4, 12, 13, 29]. However, when compared to the uninfected group, we did not find that HIV infection was associated with greater steatosis. Our observations were unexpected but few studies have included an uninfected comparison group and none have used MRS, the non-invasive gold standard to estimate steatosis. One other study of HIV-infected and uninfected men used the less sensitive liver to spleen ratio measured by CT to estimate steatosis[15] and also observed that HIV monoinfection was associated with less steatosis. That study hypothesized that HIV-monoinfected men may have less steatosis due to more frequent physician encounters, allowing for better control of risk factors associated with fatty liver disease. Another explanation for the lower liver fat in HIV-infected persons could be that HIV infection is associated with subcutaneous fat loss[30, 31] and adipocyte apoptosis in vitro[32, 33], leaving little excess fat or triglycerides to be stored in hepatocytes. We observed that both HIV-infected men and women had less abdominal and lower limb subcutaneous fat compared to uninfected men and women. It is noteworthy that compared to HIV-uninfected women, HIV-infected women were older and more likely to report being post-menopausal which has been associated with increased metabolic perturbations due to the loss of the protective effect of estrogen in studies of HIV-uninfected women; yet HIV-infected women still had less steatosis than HIV-uninfected women.
Interestingly, HIV-infected men appeared to have slightly greater VAT than uninfected men, whereas HIV-infected women had less VAT than uninfected women, which could partly explain why the difference in liver fat between HIV-infected and uninfected men was not as large as that observed between HIV-infected and uninfected women. Furthermore, HIV-infected men appeared to be less immunosuppressed and more likely to be virally suppressed than HIV-infected women, which in the modern ART era may be more likely associated with normalization of fat to that of an uninfected group, especially in the visceral compartment. After adjusting for body composition and HIV-related factors including degree of immunosuppression, viral replication, and ART use, HIV-infected men still had 25% more liver fat than HIV-infected women. By contrast, we did not observe that uninfected men had more liver fat than uninfected women contrary to prior reports[17–19, 21]. These findings suggest that the sex difference observed in HIV could be explained by other HIV-associated factors and markers such as microbial translocation and inflammation need examination.
As expected, visceral adiposity, insulin resistance, and higher ALT were associated with greater liver fat in HIV-infected and uninfected groups. In the HIV-infected group, however, the association of ALT was attenuated and no longer significantly associated after adjustment. Several studies in the general population have used ALT as a surrogate marker of steatosis[18, 19, 21], but our finding in the HIV setting indicates that ALT may not be as strong a marker of steatosis. In the HIV-infected group, we also found a strong association of higher albumin levels with greater liver fat. Low albumin levels have been associated with mortality in HIV-infected adults and thus may be a possible marker of overall health status[34]. The association of higher albumin with liver fat suggests that liver fat in virally suppressed HIV-infected persons could be an indicator of being healthy. Whether the combination of steatosis and underlying HIV-associated liver injury are associated with progression to NASH and fibrosis needs study.
Our study has some limitations. First, we examined the association of HIV, sex, and liver fat using a cross-sectional design. Second, while we pooled men and women in our analysis by HIV status because we did not observe an HIV by sex interaction, we acknowledge that the lack of an interaction may have been due to the relatively small sample size of men and women. We did not use the clinical gold standard of liver biopsy to measure liver fat, although MRS is considered the non-invasive gold standard to measure steatosis. However, because of the different timespan of data collection, some MRS data were collected on a 1.5T scanner and some on a 3T scanner, which could have biased our results. Nevertheless, data collected from the 1.5T scanner were standardized to values collected from the 3T scanner. Finally, as in all observational studies, there may have been residual or unmeasured confounders for which we were unable to control. For example, the timespan of enrollment into the VAHH and the WIHS substudy were different and could have contributed to the sex differences observed in the HIV-infected group. Many of our HIV-infected men were enrolled from a clinic setting, whereas women were originally enrolled from both clinic and non-clinical settings. However, after adjustment for demographic, lifestyle, body composition and metabolic factors, as well as indicators of receiving HIV care, we still observed that HIV-infected men had greater liver fat than HIV-infected women.
In summary, HIV-infected persons do not have greater MRS-measured liver fat than uninfected persons. Rather, HIV infection is associated with less liver fat in women, while HIV-infected and uninfected men show little difference in liver fat. Because HIV infection is associated with loss of subcutaneous fat, it is possible that there is less storage of fat in the liver to maintain subcutaneous fat. This reduction in liver fat may be enhanced in HIV-infected women, who also have lower amounts of VAT. Regardless of HIV status, visceral adiposity and insulin resistance were strongly associated with liver fat, suggesting that interventions recommended in national guidelines to reduce steatosis in the general population can be extended to the HIV population. Studies examining how HIV-associated adipose tissue changes and inflammation affect steatosis and its progression are needed.
Acknowledgments
Financial support: The Women’s Interagency HIV Study (WIHS) is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID) (U01-AI-103401, U01-AI-103408, UO1-AI-35004, UO1-AI-31834, UO1-AI-34994, U01-AI-103397, U01-AI-103390, UO1-AI-34989, and UO1-AI-42590), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA) and UL1-TR000454 (Atlanta CTSA). The study was also supported by the University of California San Francisco Liver Center [P30 DK026743], by the National Institute of Allergy and Infectious Diseases [K24 AI 108516 (PCT) and R01 AI 087176 (PCT), which was administered by the Northern California Institute for Research and Education and with resources of the Veterans Affairs Medical Center, San Francisco, CA], by the University of California, San Francisco-Gladstone Institute of Virology & Immunology Center for AIDS Research [P30-AI027763 (JCP)] and by an ACG Junior Faculty Development Award (JCP) from the American College of Gastroenterology. Data in this manuscript were collected by the WIHS. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH).
Footnotes
Author contributions:
Ani Kardashian – data interpretation, wrote half the paper, critically edited the paper, and approved the final draft of the manuscript
Yifei Ma – performed the statistical analysis, critically edited the paper, and approved the final draft of the manuscript
Rebecca Scherzer – provided expert input on the statistical analysis and interpretation of statistical data, critically edited the paper, and approved the final draft of the manuscript
Jennifer C Price – contributed MR data, assisted with data interpretation, critically edited the paper, and approved the final draft of the manuscript
Monika Sarkar – critically edited the paper and approved the final draft of the manuscript
Natalie Korn – performed the MR analysis, edited the paper, and approved the final draft of the manuscript
Kyle Tillinghast – performed the MR analysis, and approved the final draft of the manuscript
Marion Peters – provided expert input on interpretation of the data, critically edited the paper, and approved the final draft of the manuscript
Susan M. Noworolski – provided expert input on the MR analysis and interpretation of MR data, edited the paper, and approved the final draft of the manuscript
Phyllis Tien – conceived and designed the study; responsible for acquisition of data, wrote half the paper, critically edited the paper, and approved the final draft of the manuscript.
Potential competing interests: The authors do not have any commercial or other associations that might pose a conflict of interest.
References
- 1.Smith CJ, Ryom L, Weber R, Morlat P, Pradier C, Reiss P, et al. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014;384:241–248. doi: 10.1016/S0140-6736(14)60604-8. [DOI] [PubMed] [Google Scholar]
- 2.Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50:464–473. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D’Amico R, Ligabue G, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47:250–257. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 4.Hadigan C, Liebau J, Andersen R, Holalkere NS, Sahani DV. Magnetic resonance spectroscopy of hepatic lipid content and associated risk factors in HIV infection. J Acquir Immune Defic Syndr. 2007;46:312–317. doi: 10.1097/QAI.0b013e3181568cc2. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis. 2008;28:339–350. doi: 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- 6.Lazo M, Hernaez R, Eberhardt MS, Bonekamp S, Kamel I, Guallar E, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol. 2013;178:38–45. doi: 10.1093/aje/kws448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 8.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e521. doi: 10.1016/j.cgh.2011.03.020. quiz e560. [DOI] [PubMed] [Google Scholar]
- 10.Edmison J, McCullough AJ. Pathogenesis of non-alcoholic steatohepatitis: human data. Clin Liver Dis. 2007;11:75–104. ix. doi: 10.1016/j.cld.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Ghotb A, Noworolski SM, Madden E, Scherzer R, Qayyum A, Pannell J, et al. Adipose tissue and metabolic factors associated with steatosis in HIV/HCV coinfection: histology versus magnetic resonance spectroscopy. J Acquir Immune Defic Syndr. 2010;55:228–231. doi: 10.1097/QAI.0b013e3181e1d963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macias J, Gonzalez J, Tural C, Ortega-Gonzalez E, Pulido F, Rubio R, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28:1279–1287. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 13.Li Vecchi V, Soresi M, Giannitrapani L, Di Carlo P, Mazzola G, Colletti P, et al. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis. 2012;16:e397–402. doi: 10.1016/j.ijid.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Nishijima T, Gatanaga H, Shimbo T, Komatsu H, Nozaki Y, Nagata N, et al. Traditional but not HIV-related factors are associated with nonalcoholic fatty liver disease in Asian patients with HIV-1 infection. PLoS One. 2014;9:e87596. doi: 10.1371/journal.pone.0087596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Price JC, Seaberg EC, Latanich R, Budoff MJ, Kingsley LA, Palella FJ, Jr, et al. Risk factors for fatty liver in the Multicenter AIDS Cohort Study. Am J Gastroenterol. 2014;109:695–704. doi: 10.1038/ajg.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tien PC, Grunfeld C. The fatty liver in AIDS. Semin Gastrointest Dis. 2002;13:47–54. [PubMed] [Google Scholar]
- 17.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 18.Clark JM. The epidemiology of nonalcoholic fatty liver disease in adults. J Clin Gastroenterol. 2006;40(Suppl 1):S5–10. doi: 10.1097/01.mcg.0000168638.84840.ff. [DOI] [PubMed] [Google Scholar]
- 19.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 20.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–79. doi: 10.1053/gast.2003.50004. [DOI] [PubMed] [Google Scholar]
- 22.Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–327. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- 23.Volzke H, Schwarz S, Baumeister SE, Wallaschofski H, Schwahn C, Grabe HJ, et al. Menopausal status and hepatic steatosis in a general female population. Gut. 2007;56:594–595. doi: 10.1136/gut.2006.115345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang JD, Abdelmalek MF, Pang H, Guy CD, Smith AD, Diehl AM, et al. Gender and menopause impact severity of fibrosis among patients with nonalcoholic steatohepatitis. Hepatology. 2014;59:1406–1414. doi: 10.1002/hep.26761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutierrez-Grobe Y, Ponciano-Rodriguez G, Ramos MH, Uribe M, Mendez-Sanchez N. Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens Ann Hepatol. 2010;9:402–409. [PubMed] [Google Scholar]
- 26.Bacon MC, Von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clinical and diagnostic laboratory immunology. 2005;12:1013–1019. doi: 10.1128/CDLI.12.9.1013-1019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The women’s interagency HIV study. Epidemiology. 1998:117–125. [PubMed] [Google Scholar]
- 28.Noworolski SM, Tien PC, Merriman R, Vigneron DB, Qayyum A. Respiratory motion-corrected proton magnetic resonance spectroscopy of the liver. Magn Reson Imaging. 2009;27:570–576. doi: 10.1016/j.mri.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahlborg HG, Johnell O, Nilsson BE, Jeppsson S, Rannevik G, Karlsson MK. Bone loss in relation to menopause: a prospective study during 16 years. Bone. 2001;28:327–331. doi: 10.1016/s8756-3282(00)00451-8. [DOI] [PubMed] [Google Scholar]
- 30.FRAM Study Investigators. Fat Distribution in Men with HIV Infection. J Acquir Immune Defic Syndr. 40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.FRAM Study Investigators. Fat Distribution in Women with HIV Infection. J Acquir Immune Defic Syndr Hum Retrovirol. 2006;42:562–571. doi: 10.1097/01.qai.0000229996.75116.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garrabou G, Lopez S, Moren C, Martinez E, Fontdevila J, Cardellach F, et al. Mitochondrial damage in adipose tissue of untreated HIV-infected patients. AIDS. 2011;25:165–170. doi: 10.1097/QAD.0b013e3283423219. [DOI] [PubMed] [Google Scholar]
- 33.Squillace N, Bresciani E, Torsello A, Bandera A, Sabbatini F, Giovannetti C, et al. Changes in subcutaneous adipose tissue microRNA expression in HIV-infected patients. J Antimicrob Chemother. 2014;69:3067–3075. doi: 10.1093/jac/dku264. [DOI] [PubMed] [Google Scholar]
- 34.Mehta SH, Astemborski J, Sterling TR, Thomas DL, Vlahov D. Serum albumin as a prognostic indicator for HIV disease progression. AIDS Res Hum Retroviruses. 2006;22:14–21. doi: 10.1089/aid.2006.22.14. [DOI] [PubMed] [Google Scholar]