Abstract
Aim of the Study
High levels of IL-6 are believed to contribute to OA pathogenesis. Expression of IL-6 is regulated post-transcriptionally by the miR-9-MCPIP-1 axis in chondrocytes. Vorinostat (SAHA) inhibits IL-6 expression in OA chondrocytes. We investigated whether SAHA suppress the expression of IL-6 by perturbing the miR-9-MCPIP1 axis in OA chondrocytes under pathological conditions.
Materials and Methods
OA chondrocytes were isolated by enzymatic digestion and treated with IL-1β in the absence or presence of SAHA. Genes and protein expression levels were determined by TaqMan assays and Western blotting respectively. Secreted IL-6 was quantified by ELISA. MCPIP1 promoter deletion mutants were generated by PCR. Promoter recruitment of transcription factors was determined by ChIP. Nuclear run-on was employed to measure the ongoing transcription. siRNA-mediated knockdown of CEBPα expression was employed for loss of function studies.
Results
Expression of MCPIP1 was high in SAHA treated OA chondrocytes but expression of IL-6 mRNAs and secreted IL-6 were reduced by ~70%. SAHA suppressed the expression of miR-9 but enhanced the activity of the MCPIP1 promoter localized to a 156bp region which also harbors the binding site for CEBPα. Treatment with SAHA enhanced the recruitment of CEBPα to the MCPIP1 promoter. Ectopically expressed CEBPα enhanced the promoter activity and the expression of MCPIP1 while siRNA-mediated knockdown of CEBPα inhibited the expression of MCPIP1.
Conclusions
Taken together our data indicate that SAHA-mediated suppression of IL-6 expression is achieved through increased recruitment of CEBPα to the MCPIP1 promoter and by relieving the miR-9-mediated inhibition of MCPIP1 expression in OA chondrocytes.
Keywords: Vorinostat, Osteoarthritis, IL-6, MCPIP1, miRNA
Introduction
Osteoarthritis (OA) is a multifactorial disease that can be triggered and influenced by diverse factors that includes mechanical stress on the joints, socio economic, environmental and genetics of the susceptible populations [1, 2]. Current therapies are largely symptomatic and includes NSAIDs, opiates and more recently centrally acting pharmacotherapies [3, 1, 4]. IL-6 is a key cytokine that has been consistently found elevated in the serum and synovial fluids of OA patients [5, 6]. IL-6 is a potent inducer of MMP13 expression in human and bovine cartilage explants in vitro as well as in vivo in animal models of OA [7-9].
Post-transcriptional regulation of cytokine expression is mediated by coordinated action of mRNA binding proteins and microRNAs (miRNAs). MCPIP1 (monocyte chemo-attractant protein–induced protein1 (ZC3H12A; NM_025079) is an RNA binding protein with RNase activity and requires stem loop structure in the 3’UTR to cleave the target mRNA [10]. MCP-1 and IL-1β are potent inducers of MCPIP-1 in monocytes, macrophages, endothelial cells and fibroblast-like synoviocytes (FLS) [11-13]. MCPIP1 knockout mice develop normally in utero but they suffer from severe anemia and multi-organ inflammation after birth [10].
miRNAs are small 19-22 nucleotides long, non-coding RNAs that are recognized as an important regulator and fine tuner of gene expression [14]. miRNAs bind to the target mRNA that contains the “seed sequence” generally located on their 3’,5’-untranslated region (UTR) or in the coding region. Binding of miRNAs to the “seed sequence” inhibit mRNA translation or facilitates its degradation [15, 14, 16, 17]. miRNAs are first transcribed from the gene as a primary transcript and processed into functional mature miRNA by Dicer [18, 14]. Dicer null mice suffers from severe skeletal growth defects and pre-mature death indicating important role of miRNAs in skeletogenesis [19]. Expression of several miRNAs that have been shown to regulate OA related genes is dysregulated in OA [20]. MiR-140 deficient mice exhibit early onset of OA like symptoms and the onset of disease is rapid after DMM surgery [21].
Gene activation requires concerted actions of multiple factors including histone acetyltransferases (HATs) and Histone deacetylases (HDACs). HDACs removes the acetyl group from the histone and repress the gene activation [22, 23]. HDAC inhibitors (HDACi) reverse this process and have been reported to modulate pro-inflammatory cytokines. Recently Culley et al [24] reported that the broad-spectrum HDAC inhibitor Trichostatin A (TSA) protects cartilage degradation in a surgically induced mouse model of OA. In addition to TSA, Valproic Acid (VPA) and MS-275 repressed cytokine-induced expression of MMP-1 and -13 and ITF2357 reduced the production of pro-inflammatory cytokines in synovial tissues [24, 25]. Vorinostat, a class I and II HDACi has been shown to possess anti-osteoarthritic activities by inhibiting iNOS and MMPs expression [26]. Previously we demonstrated the existence of a positive feed-back loop mechanism in OA chondrocytes where cytokine-mediated upregulation of miR-9 that targets MCPIP1 3’UTR downregulates MCPIP1 expression resulting in production of high levels of IL-6 [27]. These data suggested that pharmacological upregulation of MCPIP1 in OA chondrocytes will suppress the expression of IL-6 with potential benefits in OA therapy. Therefore, we determined the effect of HDACi on the expression of MCPIP1 and IL-6 in OA chondrocytes. Our results showed that HDACi SAHA up-regulates MCPIP1 expression primarily mediated by down-regulation of miR-9 expression and upregulation of transcription factor CEBPα expression and activity. Additionally, we found that SAHA suppressed the IL-1β induced cartilage degradation in vitro, suggesting SAHA could be a promising therapeutic agent in the management of OA.
Materials and Methods
Reagents
CellGro ACTive media was purchased from CellGenix (Frieburg, Germany). DMEM media and High-Capacity cDNA Reverse Transcription Kit, TaqMan Gene Expression Assay, miR-9 mimics and siRNA against CEBPα were procured from Life Technologies (Carlsbad, CA, USA). For cartilage digestion pronase and collagenase were obtained from Roche Diagnostics (Indianapolis, IN, USA). Qiazol and miRNeasy kit were procured from Qiagen (Valencia, CA, USA). Recombinant human IL-1β was obtained from R&D Systems (Minneapolis, MN, USA). Antibodies against ZC3H12A (GenTex, Irvine, CA, USA), β-ACTIN, CEBPα and MMP-13 (Santa Cruz Biotechnology, Dallas, TX, USA) and IL-6, Ac-H3, Ac-H4, H3 and H4 were obtained from Cell Signaling Technology (Danvers, MA, USA). Horseradish peroxidase conjugated anti-mouse and anti-rabbit secondary antibodies were obtained from Pierce Biotechnology (Rockford, IL, USA). pcDNA3.1(−) rat C/EBP alpha was a gift from Peter Johnson (Addgene plasmid # 12550). HDAC inhibitors SAHA, TSA, VPA and MS-275 were purchased from Selleckchem (Houston, TX, USA).
Cartilage and chondrocytes isolation
The study protocol was approved by the Institutional Review Board of North East Ohio Medical University, Rootstown, OH, as “exempt and not a human subject study”. The OA cartilage samples used were discarded tissues obtained from donors who underwent the total knee arthroplasty (n=8, mean age, 56±12.2 years; 4 males and 4 females). Cartilage pieces were carefully excised macroscopically from smooth (modified outerbridge grade I) and damaged areas (modified outerbridge grade III) and immediately stored in liquid nitrogen for later use or, for chondrocytes preparation cartilage pieces were sequentially digested in DMEM (10%FBS) with Pronase (1 mg/ml) for 1 hr followed by digestion with Collagenase (1 mg/ml) for overnight essentially as described previously [17, 28].
RNA Isolation and TaqMan Assays
Control or treated cartilage samples were grinded with mortar and pestle in liquid nitrogen. Powdered cartilage was immediately transferred into Qiazol. Solution was briefly vortexed and chloroform was added. After centrifugation, aqueous phase was subsequently transferred into new tube and ethanol was added and vortexed. Mix was pipeted into RNeasy Mini Spin column. Subsequently RNA isolation procedure was followed according to the manufacturer’s recommendations and total RNA including small RNA was prepared using miRNeasy kit as recommended by the manufacturer. RNA quality and quantity was determined by NanoDrop 2000c (Thermofisher). For mRNA expression analyses, cDNA was synthesized from 1.0 μg total RNA using High-Capacity cDNA Reverse Transcription Kit (Thermofisher). Real-time PCR reaction was carried out using TaqMan Gene Expression Assays according to the manufacturer's instructions. Expression of mature microRNA was quantified using TaqMan MicroRNA Assays (Life Technology, Carlsbad, CA, USA). microRNA-9 or RNU6B from control or treated samples were reverse transcribed using respective stem loop RT primer from Life Technology/Thermofisher, 10X RT buffer, 10 mM each dNTP, 50 units MultiScribe reverse transcriptase, and 20 units of RNase inhibitor. Expression levels were determined in one plate for all samples simultaneously and normalized to the corresponding amounts of β-ACTIN or RNU6B cDNA. Relative expression levels were calculated using the 2–ΔΔCT method.
Cartilage explants and Glycosaminoglycan (GAG) Assay
Equal sizes of cartilage slices were dissected from the patients samples using scalpel blade, weighed and distributed equally in wells of a 24 well culture plate (n=9; 18.6±3.1mg/well). Cartilage slices were cultured for 24 hrs in using DMEM supplemented with 10% FBS. Medium was changed to CellGro ACTive medium supplemented with IL-1β (1.0ng/ml) alone. Post two hrs induction samples were left untreated or supplemented with SAHA (1.0μM). At day one or day 4 culture supernatant was collected and sulphated glycosaminoglycan release into the medium upon treatment with IL-1β or SAHA was quantified using 1,9 dimethylmethylene blue (DMMB) assay (Astarte Biologics, Bothell, WA). Chondroitin sulphate (Sigma) was used to generate the standard curve to measure the GAG content. Samples were diluted as necessary to bring them into the range of standard curve. Absorbance was read at 595nm and the GAG content was normalized to explant wet weight.
Chondrocyte Treatment and Transfection
For each treatment 1.2 million primary chondrocytes were seeded in 35 cm dishes in CellGro ACTive medium. Cells were first induced with 2 ng/ml IL-1β for 2 hrs and then treated with HDAC inhibitors (TSA; 500nM, SAHA; 1μM, VPA; 1mM and MS-275; 1μM) for additional 14 hrs. After treatment cells were washed and RNA or protein was prepared immediately or cells were stored at −80°C for later use. Chondrocyte culture supernatants were collected as required and stored at −80°C prior to use in ELISA assay. For CEBPα siRNA or miR-9 overexpression study 5 million cells were seeded in 10 cm petridishes. Chondrocytes were transfected with miR-9 mimic or siRNA at a final concentration of 100nM using Amaxa Nucleofactor kit according to the manufacturers recommendations. In brief, seeded chondrocytes were digested with 1mg/ml Pronase and Collagenase for 2-3 hrs. miR-9 mimic or siRNA was diluted into 100 μl nucleofactor solution and chondrocytes were transfected using Amaxa Nucleofactor System electroprogramme P01. Cells were diluted in DMEM supplemented with 10% FBS and 1.5 million chondrocytes were seeded for RNA or protein isolation respectively. Twenty hrs later medium was changed to CellGro ACTive medium and treatments were performed in CellGro ACTive medium. Total RNA or protein was isolated after 16 hrs of treatment. For MCPIP1 3’UTR luciferase activity chondrocytes were transfected using wild type or miR-9 mutant luciferase reporter construct as above. Hundred thousand chondrocytes were seeded into a 12 well plate in duplicate and were treated as required and dual luciferase activity assay was performed according to manufacturer’s recommendations using the Dual Glo luciferase assay kit (Promega, WI, USA). For promoter activity analysis, full length or deleted promoter reporter constructs were transfected as described previously (17) and seeded into a 12 well plate. After treatment luciferase activity assay was performed using the Dual Glo luciferase kit. For the CEBPα knockdown studies, OA chondrocytes were transfected with OnTarget plus siRNAs (# L-006422-00-0005, Dharmacon) as described above and the mRNA expression was assessed 36 hours later to verify the knockdown.
Luciferase Activity Assay
For promoter analysis OA chondrocytes were transfected with the PCR generated MCPIP1 promoter fragments cloned into a luciferase reporter vector or with CEBPα expression plasmid (Addgene plasmid # 12550). Transfected OA chondrocytes were stimulated with IL-1β in the presence or absence of SAHA. Dual luciferase activity assay was performed according to the manufacturer’s recommendations using the Dual Glo Assay kit (Promega) [17].
Immunoblot Analyses
After desired treatment chondrocytes were harvested, washed with PBS and lysed in ice-cold protein lysis buffer (50 mM Tris pH 7.6, 400 mM NaCl, 0.5% NP-40, 1 mM PMSF and 1x protease inhibitor cocktail (Roche, Mannheim, Germany) [17, 29]. Lysates were clarified by centrifugation (15000g for 10 min at 4°C) and the supernatant was analyzed immediately or stored at −80°C. Equivalent amounts of proteins were resolved by 10% SDS–PAGE and transferred onto a PVDF membrane (Hybond P, Amersham Biosciences, Piscataway, New Jersey, USA). After blocking in 5% milk, membranes were incubated with anti-IL-6 (1:1000), anti-MCPIP1 (1:1000) or anti-β-Actin (1:5000) primary antibodies overnight at 4°C. To detect acetylated histone, cells were lysed in protein loading buffer (0.313 M Tris pH 6.8, 10%SDS, 40% glycerol, 0.05% bromophenol blue and 2% β-ME). Cells were sonicated three times for 10s each and separated on 12% SDS-PAGE. After blocking membranes were incubated with anti-Ac-H3 (1:1000), anti-Ac-H4 (1:1000), anti-H3 (1:1000) or anti-H4 (1:1000) antibodies overnight at 4°C. Every primary antibody incubation was followed by horseradish peroxidase-conjugated secondary antibody (1:5000). Western blots were visualized using Syngene Pxi imager. Quantification of immuno-reactive bands was performed using the Gene Tools Software (Syngene, Frederick, MD, USA).
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described previously [29]. Briefly a total of 1 × 106 human OA chondrocytes were treated with DMSO or SAHA in the presence of IL-1β. After cross-linking chondrocytes were suspended in 250 μl SDA lysis buffer followed by sonication to an average DNA length of 200–1000 bp. Cleared samples were diluted in a ChIP dilution buffer and anti-CEBPα antibody (Santa Cruz Biotechnology) was added to each tube. The protein A/G beads were washed with low salt immune complex wash buffer, high salt immune complex wash buffer and LiCl immune complex wash buffer. The protein-DNA complex was eluted with 250 μl of elution buffer. After purification DNA was quantitated by qPCR using primer pairs: 5’GAGGAGCGGAGCAGGAAG-3’ (Forward) and 5’-CGAGTCCTGGGGGTAAGG-3’ (Reverse).
Nuclear Run-on Assay
Non-radioactive nuclear run on assay was performed essentially as described by Kevin et al [30]. Assay was coupled with qRT-PCR to quantitate the rate of transcription. In brief, after treatment chondrocyte pellet was resuspended in lysis buffer and then passed through a 21-gauge needle. Nuclei were collected by centrifugation at 15000g for 10 min at 4°C. The run-on reaction was carried out in a 2X transcription buffer. The reaction was commenced with the addition of 4mM biotin-16-UTP (Roche Applied Science) and incubated for 1.0 hrs at 30°C. RNA isolation was done using QIAzol reagent. Magnetic beads (Dynabeads M-280, Dynal, Oslo, Norway) that were covalently linked to streptavidin were used to isolate the biotin-labeled-run on RNA. The beads were resuspended in RNA free water. Reverse transcription and TaqMan real time PCR was performed as described previously [17].
Statistical Analyses
The values given are means ± S.E. The significant difference between the experimental groups and controls was assessed by Student's t test. Each experiment was repeated three times using three independent patient’s samples. The difference was considered significant if the p value was <0.05.
Results
IL-1β induced cartilage degradation in vitro was suppressed by the histone deacetylase inhibitor (HDACi) SAHA
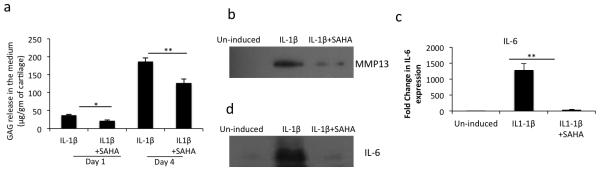
Although, the use of histone deacetylase inhibitors (HDACi) has emerged as a potential therapeutic strategy for different diseases and HDACi have displayed chondroprotective effects in vitro and in animal model studies [31, 32, 26] such studies with SAHA, a class-I and II HDACi, have not been reported. We used an in vitro model of cartilage matrix degradation to assess the impact of SAHA on the sulphated glycosaminoglycan (GAG) release in the culture media in the presence or absence of IL-1β. Treatment with SAHA resulted in significantly decreased GAG release in IL-1β-stimulated human cartilage explants as compared to cartilage explants treated with IL-1β alone (Fig. 1a). These data suggests that treatment with SAHA inhibited the expression of enzymes involved in cartilage matrix breakdown. As MMP-13 is reported to degrade matrix collagens and aggrecans and is induced upon stimulation with IL-1β, we next quantified the expression level of MMP-13 in culture medium of human cartilage explants stimulated with IL-1β with or without SAHA and found that the expression of MMP-13 was significantly reduced in the presence of SAHA (Fig. 1b). Gene and protein expression analyses also showed reduced expression of IL-6 mRNA (Fig. 1c) and protein level (Fig. 1d) in culture supernatants of human cartilage explants treated with SAHA+ IL-1β compared to the cartilage explants treated with IL-1β alone. This suggests that, at least in part, the cartilage protective effect could be mediated via inhibition of IL-6 and MMP-13 expression.
Figure 1. IL-1β- induced OA cartilage degradation was suppressed by SAHA in vitro.
(a) Cartilage explants were carefully dissected and cultured in CellGro ACTive medium and treated with IL-1β in the presence or absence of 1μM SAHA for the indicated time points. Supernatant was collected and GAG content was measured by a commercially available kit (#8000, Astarte Biologics). (b) and (d) Supernatant from control and treated cartilage explants was used to perform the Western Blotting using anti-MMP-13 (#sc-30073, Santa Cruz Biotechnology) and anti-IL-6 (#12153, Cell Signaling) antibody. (c) Control or treated cartilage explants were used to isolate total RNA and TaqMan assay was employed to quantify the expression of IL-6 mRNA. Bars represent ±SD of independent experiments using five patients’ cartilage samples (*P <0.05; **P <0.005; paired student t-test).
SAHA, but not VPA, induce MCPIP1 expression and suppresses IL-6 expression in human OA chondrocytes
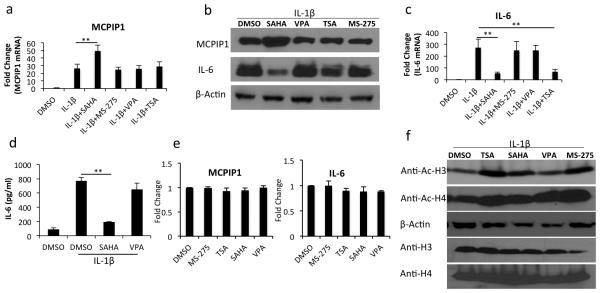
Chondrocyte is the only cell type present in the cartilage and MCPIP1 has been shown by us to be a negative regulator of IL-6 expression in OA chondrocytes [27]. Therefore, we tested whether induction of MCPIP1 and reciprocal down-regulation of IL-6 expression is a generalized effect of HDACi or a specific effect of SAHA. We found that SAHA, but not other HDACi tested, significantly upegulated the MCPIP1 gene expression with a concomitant increase in MCPIP1 protein levels in OA chondrocytes stimulated with IL-1β as measured by TaqMan assays and Western blotting respectively (Fig. 2a, -2b). IL-1β-induced upregulation of IL-6 mRNA and protein was reduced approximately 80-90% in SAHA-treated OA chondrocytes (Fig. 2b and -2c). We also quantified the levels of secreted IL-6 protein in the culture supernatants using a specific ELISA and the results demonstrated a significant reduction of secreted IL-6 protein in SAHA treated OA chondrocytes but not in VPA treated OA chondrocytes (Fig. 2d). Next we determined the effect of HDACi on the basal expression of MCPIP1 and IL-6 genes and the data demonstrated that HDACi tested did not induce the expression of MCPIP1 and IL-6 in OA chondrocytes (Fig. 2e). As a control experiment we also determined the acetylation of histone H3 and H4 treated with IL-1β and the HDACi by Western blotting and the results showed enhanced acetylation of both histones compared to control (Fig. 2f). These findings demonstrate that induction of MCPIP1 and suppression of IL-6 expression is not a pan-HDACi effect but was a specific effect of SAHA in human OA chondrocytes under pathological conditions. Based on this data we used SAHA in all of the subsequent studies described here.
Figure 2. SAHA induces MCPIP1 expression and downregulates IL-6 expression in IL-1β induced OA chondrocytes.
(a) and (c) Chondrocytes were induced with 2 ng/ml IL-1β for 2 hrs and then treated with different HDAC inhibitors (SAHA, 1μM, MS-275 1μM, VPA 1mM and TSA 500nM) for additional 14 hrs in serum free CellGro Active chondrocyte culture medium. Chondrocytes were harvested and RNA was prepared. MCPIP1 and IL-6 expression was measured by TaqMan real time PCR. (b) Chondrocytes were treated as in (a). Chondrocyte lysates were resolved on SDS PAGE and MCPIP1 and IL-6 protein levels were measured using anti-MCPIP1 antibody (# GTX110807, GeneTex) or anti-IL-6 antibody (#12153, Cell Signaling) specific antibodies. (d) Culture supernatants from the control or treated OA chondrocytes were used to quantitate the secreted IL-6 protein levels by ELISA. (e) Chondrocytes were cultured in CellGro Active medium containing serum and treated with different HDAC inhibitors. Sixteen hrs later chondrocytes were harvested and basal expression of MCPIP1 and IL-6 mRNAs was quantified by TaqMan assays. (f) Chondrocytes were induced with 2 ng/ml of IL-1β for 2 hrs and then treated with different HDAC inhibitors (SAHA, 1μM, MS-275 1μM, VPA 1mM and TSA 500nM) for additional 14 hrs in serum free CellGro Active chondrocyte culture medium. Chondrocytes were harvested and lysates were prepared in 2X protein lysis buffer. Western blotting was performed using anti-acetyl H3 (# 9649, Cell Signaling) or H4 antibody (# 9672, Cell Signaling). Expression level of β-actin (# sc-130656, Santa Cruz Biotechnology), total histone H3 (# 9715, Cell Signaling) or H4 (# 2592, Cell Signaling) was used to perform the normalization. Bars represent ±SD from three independent experiments (*P <0.05; **P <0.005; paired student t-test).
SAHA downregulated the IL-1β-induced miR-9 expression in OA chondrocytes and in cultured OA cartilage explants
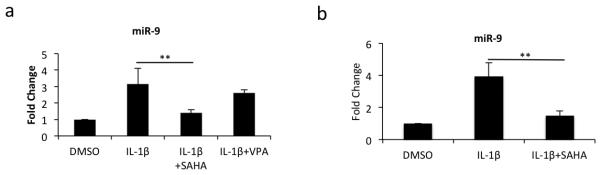
Since MCPIP1 mRNA is a target of miR-9 which inhibits its expression [27] we determined whether upregulation of MCPIP1 expression in OA chondrocytes and cartilage explants treated with SAHA was mediated via modulation of miR-9 expression in OA chondrocytes and cartilage explants. Gene expression analyses showed that the expression of miR-9 was reduced by ~60% in SAHA treated OA chondrocytes as compared to OA chondrocytes treated with IL-1β alone (Fig. 3a). However, and importantly this robust inhibitory effect on the expression of miR-9 was not observed in OA chondrocytes treated with VPA (Fig. 3a) again suggesting a rather specific effect of SAHA in this regard. In SAHA treated cartilage explants also, expression of miR-9 was reduced ~2 fold (Fig. 3b). Since miR-9 is a negative regulator of MCPIP expression, our results indicated that downregulation of miR-9 expression in OA chondrocytes by SAHA may play a role in the upregulation of MCPIP1 expression under pathological conditions.
Figure 3. SAHA downregulates miR-9 expression in OA chondrocytes and in cultured OA cartilage explants.
(a) Chondrocytes or (b) cartilage explants were stimulated with IL-1β (2ng/ml; n=3) in the presence or absence of SAHA (1mM; n=5) for 16 hrs or 4 days. Total RNA was harvested and miR-9 expression was measured by TaqMan assay. Expression of RNU6B was used as a normalizing control. (*P <0.05; **P <0.005; paired student t-test).
Expression of MCPIP1 was suppressed in SAHA treated OA chondrocytes upon miR-9 overexpression under pathological conditions
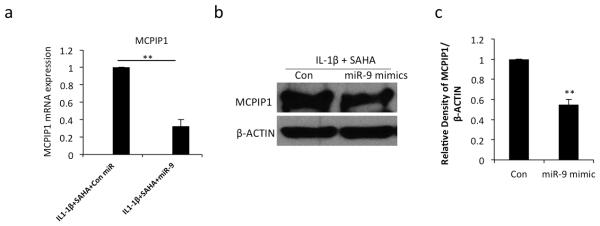
Data presented above raised the intriguing possibility that inhibition of miR-9 expression was an important event in the SAHA-mediated upregulation of MCPIP1 expression. To explore this further we transfected OA chondrocytes with control or miR-9 mimics and then treated the OA chondrocytes with SAHA and IL-1β. We observed ~40% reduction in MCPIP1 mRNA expression and a 1.4 fold reduction in MCPIP1 protein level in OA chondrocytes with overexpression of miR-9 (Fig. 4a, -4b-c). These data indicated that the suppression of miR-9 expression was important in the upregulation of MCPIP1 expression in SAHA treated OA chondrocytes under pathological conditions.
Figure 4. Overexpression of miR-9 suppress MCPIP1 expression in IL-1β stimulated and SAHA treated OA chondrocytes.
(a) Chondrocytes were transfected with control miR or miR-9 mimic (200nM) using Nucleofactor kit and were grown for 24 hrs and then treated with IL-1β (2ng/ml) and SAHA (1 μM). After 16 hrs of treatment total RNA was isolated and MCPIP1 expression was measured with β-actin as a normalizing control. (b) Chondrocytes were treated as above and subjected to Western blot analysis to detect the MCPIP1 and IL-6 proteins. β-actin was used as a loading control. Representative blot is shown. (c) Densitometric analysis of immunoreactive bands represented in (b) was performed. (*P <0.05; **P <0.005; paired student t-test).
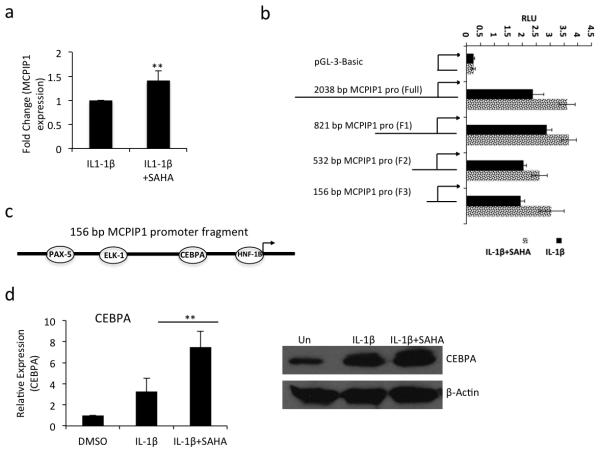
Treatment with SAHA enhanced the MCPIP1 mRNA expression in OA chondrocytes
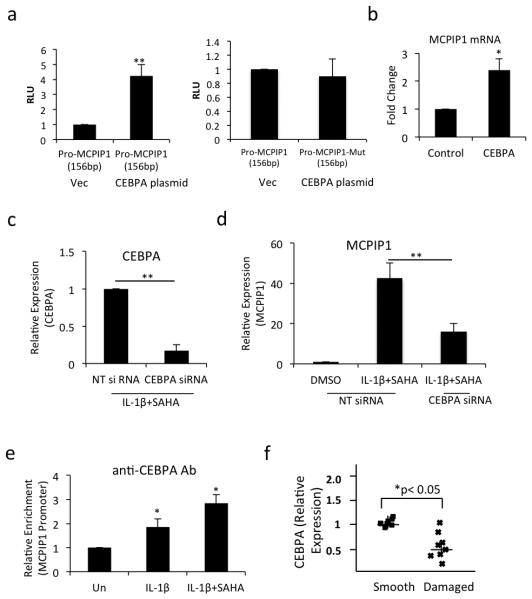
HDAC inhibitors suppress the de-acetylation status of the chromatin, which affect the transcription of the genes [22]. Since upregulation of MCPIP1 expression may also be regulated at the transcriptional level upon SAHA treatment we tested this hypothesis by performing nuclear run-on assay. Purified nuclear RNA was subjected to RT-qPCR and showed a 1.4 fold-increased expression of MCPIP1 mRNAs in IL-1β + SAHA treated OA chondrocytes as compared to OA chondrocytes stimulated with IL-1β alone (Fig. 5a). Furthermore, OA chondrocytes transfected with the 2083 bp MCPIP1 promoter activity reporter construct showed enhanced luciferase activity in IL-1β + SAHA treated OA chondrocytes (Fig. 5b). To delineate the genomic area in the promoter region responsible for the increased promoter activity, OA chondrocytes were transfected with sequential deletion mutants of MCPIP1 promoter and luciferase activity was assayed upon IL-1β + SAHA treatment. All the deletion constructs including the smallest 156bp promoter DNA fragment showed enhanced luciferase activity upon SAHA treatment suggesting that this 156bp region of the DNA contained the potential transcription factor(s) binding site (Fig 5b). By in silico analysis using PROMO transcription factor binding tool [33] we identified the binding sites for PAX-5, ELK-1, CEBPα and HNF-1B transcription factors within the 156 bp region of the MCPIP1 promoter (Fig. 5c). We quantitated the expression of PAX-5, ELK-1, CEBPα and HNF-1B and discovered significantly increased expression of CEBPα in IL-1β + SAHA treated compared to IL-1β alone treated OA chondrocytes (Fig. 5d, P<0.05) suggesting that promoter recruitment of CEBPα may be responsible for the enhanced expression of MCPIP1 upon treatment with SAHA. To test this hypothesis we co-transfected the OA chondrocytes with the wild type 156bp MCPIP1 promoter fragment or a 156bp MCPIP1 promoter fragment with mutated CEBPα binding site with either an empty vector or with the CEBPα overexpression plasmid. Transfected OA chondrocytes were then treated with IL-1β + SAHA as above and the Luciferase enzyme activity was measured. Luciferase activity was significantly enhanced in CEBPα overexpressing OA chondrocytes but was abrogated in OA chondrocytes transfected with the promoter vector containing the mutated CEBPα binding site (Fig. 6a). Furthermore, we also found that the endogenous MCPIP1 mRNA expression was enhanced upon overexpression of CEBPα without exogenous stimulus (Fig. 6b). We also performed loss of function experiment using siRNA-mediated depletion of CEBPα in OA chondrocytes. We observed approximately 80% knockdown of CEBPα in siRNA-transfected OA chondrocytes (Fig 6c). Expression of MCPIP1 was significantly abolished in CEBPα depleted chondrocytes compared to the OA chondrocytes transfected with non-targeted siRNAs (Fig 6d). These data indicate that induction of MCPIP1 mRNA expression upon SAHA treatment is CEBPα-dependent. Although knockdown of CEBPα could not reduce the MCPIP1 mRNA expression to the basal level suggesting that other factors are also involved in the induction of MCPIP1 expression upon SAHA treatment in OA chondrocytes. Next we evaluated the CEBPα binding to the MCPIP1 promoter region by ChIP assay. Our ChIP assay data showed an approximately 1.5 fold increased binding of CEBPα to the MCPIP1 promoter in IL-1β + SAHA treated OA chondrocytes compared to OA chondrocytes treated with IL-1β alone (Fig. 6e). Finally we found a significant reduction in CEBPα expression in damaged OA cartilage compared to the smooth cartilage of the same patient (Fig. 6f) which suggests that low level of MCPIP1 expression in OA cartilage could be due to the low expression of CEBPα in chondrocytes in the damaged OA cartilage.
Figure 5. Transcriptional activation of MCPIP1 by SAHA in OA chondrocytes.
(a) OA chondrocytes were stimulated with IL-1β (2ng/ml) in the presence or absence of SAHA (1μM) for 16 hrs. Chondrocytes were harvested and nuclei were isolated and subjected to nuclear run on assay as described above. Total RNA was isolated and MCPIP1 expression was quantitated using TaqMan assay (n=3). (b) OA chondrocytes were transfected with full length or deletion mutants of the MCPIP1 promoter reporter constructs and were stimulated with IL-1β alone or IL-1β + SAHA. Luciferase activity was measured after 24 hrs of stimulation. Renilla luciferase was used to normalize the transfection efficiency. (c) Diagramatic representation of the the156bp MCPIP1 promoter fragment with predicted transcription factors binding sites. (d) Transcription factor CEBPα was upregulated upon IL-1β and SAHA treatment determined by TaqMan assay and Western blot analysis. (*P <0.05; **P <0.005; paired student t-test).
Figure 6. CEBPα upregulates MCPIP1 expression in OA chondrocytes.
(a) OA chondrocytes were co-transfected with 156bp MCPIP1 wild type or mutant luciferase construct and CEBPα expression plasmid. Luciferase activity was measured and normalized against Renilla luciferase. (b) Endogenous MCPIP1 mRNA was measured by qPCR upon transfection of CEBPα in OA chondrocytes. (c) OA chondrocytes were transfected with non-targeted siRNA or siRNA against CEBPα. Twenty hours later chondrocytes were induced with 2 ng/ml IL-1β for 2 hrs and then treated with SAHA for an additional 14 hrs in serum free CellGro Active chondrocyte culture medium. Expression of CEBPα was determined using TaqMan Assay. (d) MCPIP1 expression was quantified by TaqMan Assay in OA chondrocytes transfected with non-targeted and CEBPα targeting siRNAs. (e) OA chondrocytes treated with IL-1β alone or with SAHA were subjected to ChIP analysis using anti-CEBPα antibody (# sc-61, Santa Cruz Biotechnology). Immunoprecipitated DNA was quantitated by qPCR and the values plotted against immunoprecipitated DNA from un-induced OA chondrocytes. (f) Expression of CEBPα mRNA was measured in smooth and damaged cartilage by TaqMan assay. (*P <0.05; **P <0.005; paired student t-test).
Discussion
Until recently OA was thought of mostly as due to “wear and tear” leading to cartilage degradation in the affected joints but now the focus has shifted to the concept that inflammation is a critical mediator of OA pathogenesis. Inflammatory mediators including cytokines and matrix degrading proteases are induced via the activation of several different pathways whose combined activities play a role in OA pathogenesis. In this regard inhibition of effector molecules may have beneficial impact on the lives of OA patients. In a previous study we have shown that MCPIP1 is a negative regulator of IL-6 expression in human OA chondrocytes under pathological conditions. In the present study we analyzed the impact of a class 1 and II HDACi SAHA on cartilage matrix degradation and expression of inflammatory mediators under pathological conditions using an in vitro model of cartilage inflammation. Interestingly, we found that under pathological conditions human OA cartilage explants in the presence of SAHA released significantly less sGAG in the culture media indicating that SAHA suppress the cytokine-induced matrix degradation in vitro. We also show that treatment with SAHA enhanced the expression of MCPIP1 and downregulated the expression of IL-6 and MMP-13 in IL-1β stimulated human cartilage explants and OA chondrocytes. The enhanced expression of MCPIP1 was achieved by two independent mechanisms. First expression of miR-9, which is a negative regulator of MCPIP1 expression, was downregulated upon SAHA treatment in IL-1β-stimulated OA chondrocytes. Second, we discovered that the transcription factor CEBPα is a critical mediator of MCPIP1 expression and is suppressed in human OA chondrocytes under pathological conditions. However, its expression was rescued by treatment with SAHA in OA chondrocytes and explains the observed enhanced expression of MCPIP1.
IL-6 is a pleiotropic cytokine, possessing pro- and anti-inflammatory properties [34]. IL-6 is a key mediator of induction of multiple inflammatory mediators, including MMP13, in human and bovine cartilage explants as well as in mouse model of human OA [35, 36]. IL-6 also inhibits the expression of type II collagen building block of cartilage [7, 37, 9]. Therefore, inhibition of IL-6 expression in OA could be an important therapeutic strategy in developing more effective OA management strategies. While suppression of IL-6 expression upon HDACi treatment has been demonstrated earlier [38], the mechanism was not extensively evaluated thus leaving gaps in our knowledge. We investigated the mechanism of IL-6 suppression by SAHA and for the first time demonstrate a link between the upregulation of MCPIP1 and inhibition of IL-6 expression in OA chondrocytes treated with SAHA under pathological conditions.
Histone deacetylases are important determinant of gene expression and regulate their expression by targeting histone or non-histone proteins [39]. Studies suggest that global HDAC activities are increased in RA patients when compared to healthy individuals [40]. Additionally, in the CAIA or DMM mouse model of OA, TSA treatment effectively protected the cartilage [41, 24]. Valproic acid, TSA, and MS-275 block the cytokine-induced expression of MMP1 and MMP13 in human articular chondrocytes [24]. In the ACLT animal model of OA, animals treated with TSA showed less cartilage degradation compared to controls [31] but the mechanism of the cartilage protective effect was not described. In this study we provide strong evidence that SAHA inhibit the expression of MMP-13 and further enhance the IL-1β-mediated induction of MCPIP1 expression. MCPIP1 is an important negative post-transcriptional regulator of IL-6 expression [42] and directly suppress its expression and indirectly of IL-6 inducible gene MMP-13 in OA chondrocytes and cartilage explants. Importantly, the effect on MCPIP1 expression is not a generalized effect of the class1 HDACi as VPA and MS-275 were not effective in rescuing the expression of MCPIP1 in OA chondrocytes under pathological conditions.
Transcription factor CEBPα belongs to a family of basic leucine zipper transcription factors with 5 additional members (CEBPα,-β,-γ,-δ,-ε and -ζ). Members of CEBP family play a pivotal role in regulating lineage commitment, growth, differentiation and inflammatory response [43-45]. CEBPβ has been shown to repress COL2A1 expression and induce terminal differentiation of chondrocytes by activating MMP13, Vegf and Runx2 [46, 47]. Recently it was shown that exposure of B cells to a pulse of CEBPα rapidly and efficiently reprogrammed the cells into iPSCs by Yamanaka factors OSKM [52]. However, role of CEBPα has not been clearly established in OA although various studies have shown cartilage related gene regulation by CEBPβ in chondrocytes [48, 46, 47, 49]. Recently CEBPα expression in the cartilage was demonstrated and it was found that mature chondrocytes express CEBPα [50]. We show here that the CEBPα expression in OA chondrocytes prepared from the damaged OA cartilage was significantly downregulated compared to the levels detected in chondrocytes prepared from smooth cartilage of the same patient which suggests that CEBPα may play a role in OA pathogenesis. Recently Okuma T et. al. [51] performed microarray analysis and demonstrated that when dominant negative form of CEBPA was overexpressed in ATDC5 cells, expression of Zc3h12a was repressed, suggesting that CEBPA activity may be important for the expression of Zc3h12a. This supports our data reported here demonstrating that indeed CEBPA positively regulates MCPIP1 expression in human OA chondrocytes. In this study we for the first time identified a link between the expression of CEBPα, MCPIP1 and IL-6 expression in OA chondrocytes. We propose that HDAC inhibitor SAHA increase the transcriptional activity of CEBPα to promote MCPIP1 expression which post-transcriptionally regulate the expression of IL-6 in OA chondrocytes. Suppression of CEBPα expression in OA cartilage perturb the MCPIP1 gene expression that may contribute to the known overexpression of IL-6 and the associated pathophysiology in OA joints.
There is a need to develop better and more effective therapeutic strategies for the management of OA. Our data provide new insights into SAHA functionality in OA chondrocytes and provide a direction for future work that may lead to the development of innovative therapeutic strategies for the management of OA.
Acknowledgements
This work was supported in part by USPHS/NIH grants (RO1 AT007373, RO1 AT005520, RO1 AR067056, R21 AR064890) and funds from North East Ohio Medical University to TMH.
Footnotes
Declaration of Interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Contributor Information
Mohammad S Makki, Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio 44272.
Tariq M Haqqi, Department of Anatomy and Neurobiology, Northeast Ohio Medical University, Rootstown, Ohio 44272.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatol. 2012;64:1697–707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bian Q, Wang YJ, Liu SF, Li YP. Osteoarthritis: genetic factors, animal models, mechanisms, and therapies. Front Biosci. 2012;4:74–100. doi: 10.2741/361. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheumatol. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandi ML, Gennari L, Cerinic MM, Becherini L, Falchetti A, Masi L, Gennari C, Reginster JY. Genetic markers of osteoarticular disorders: facts and hopes. Arthritis Res. 2001;3:270–80. doi: 10.1186/ar316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldring SR, Goldring MB. The role of cytokines in cartilage matrix degeneration in osteoarthritis. Clin Orthop Relat Res. 2004;427(Suppl):S27–36. doi: 10.1097/01.blo.0000144854.66565.8f. [DOI] [PubMed] [Google Scholar]
- 6.Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, Spector TD. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: The Chingford Study. Arthritis Rheumatol. 2009;60:2037–45. doi: 10.1002/art.24598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cawston TE, Curry VA, Summers CA, Clark IM, Riley GP, Life PF, et al. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheumatol. 1998;41:1760–71. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Houssiau FA, Devogelaer JP, Van Damme J, de Deuxchaisnes CN, Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheumatol. 1988;31:784–8. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- 9.Poree B, Kypriotou M, Chadjichristos C, Beauchef G, Renard E, Legendre F, Melin M, Gueret S, Hartmann DJ, Malléin-Gerin F, Pujol JP, Boumediene K, Galéra P. Interleukin-6 (IL-6) and/or soluble IL-6 receptor down-regulation of human type II collagen gene expression in articular chondrocytes requires a decrease of Sp1.Sp3 ratio and of the binding activity of both factors to the COL2A1 promoter. J Biol Chem. 2008;283:4850–65. doi: 10.1074/jbc.M706387200. [DOI] [PubMed] [Google Scholar]
- 10.Matsushita K, Takeuchi O, Standley DM, Kumagai Y, Kawagoe T, Miyake T, Satoh T, Kato H, Tsujimura T, Nakamura H, Akira S. Zc3h12a is an RNase essential for controlling immune responses by regulating mRNA decay. Nature. 2009;458:1185–90. doi: 10.1038/nature07924. [DOI] [PubMed] [Google Scholar]
- 11.Zhou L, Azfer A, Niu J, Graham S, Choudhury M, Adamski FM, Younce C, Binkley PF, Kolattukudy PE. Monocyte chemoattractant protein-1 induces a novel transcription factor that causes cardiac myocyte apoptosis and ventricular dysfunction. Circ Res. 2006;98:1177–85. doi: 10.1161/01.RES.0000220106.64661.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koga T, Yamasaki S, Migita K, Kita J, Okada A, Kawashiri S, Iwamoto N, Tamai M, Arima K, Origuchi T, Nakamura H, Osaki M, Tsurumoto T, Shindo H, Eguchi K, Kawakami A. Post-transcriptional regulation of IL-6 production by Zc3h12a in fibroblast-like synovial cells. Clin Exp Rheumatol. 2011;29:906–12. [PubMed] [Google Scholar]
- 13.Mizgalska D, Wegrzyn P, Murzyn K, Kasza A, Koj A, Jura J, Jarzab B, Jura J. Interleukin-1-inducible MCPIP protein has structural and functional properties of RNase and participates in degradation of IL-1beta mRNA. FEBS J. 2009;276:7386–99. doi: 10.1111/j.1742-4658.2009.07452.x. [DOI] [PubMed] [Google Scholar]
- 14.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 15.Farh KK, Grimson A, Jan C, Lewis BP, Johnston WK, Lim LP, Burge CB, Bartel DP. The widespread impact of mammalian MicroRNAs on mRNA repression and evolution. Science. 2005;310:1817–21. doi: 10.1126/science.1121158. [DOI] [PubMed] [Google Scholar]
- 16.Krol J, Loedige I, Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nat Rev Genet. 2010;11:597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 17.Akhtar N, Makki MS, Haqqi TM. MicroRNA-602 and MicroRNA-608 Regulate Sonic Hedgehog Expression via Target Sites in the Coding Region in Human Chondrocytes. Arthritis Rheumatol. 2015;67(2):423–34. doi: 10.1002/art.38952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tetreault N, De Guire V. miRNAs: their discovery, biogenesis and mechanism of action. Clin Biochem. 2013;46:842–5. doi: 10.1016/j.clinbiochem.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Lu J, Cobb BS, Rodda SJ, McMahon AP, Schipani E, et al. Dicer-dependent pathways regulate chondrocyte proliferation and differentiation. Proc Natl Acad Sci U S A. 2008;105:1949–54. doi: 10.1073/pnas.0707900105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyaki S, Asahara H. Macro view of microRNA function in osteoarthritis. Nat Rev Rheumatol. 2012;8:543–52. doi: 10.1038/nrrheum.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyaki S, Sato T, Inoue A, Otsuki S, Ito Y, Yokoyama S, Kato Y, Takemoto F, Nakasa T, Yamashita S, Takada S, Lotz MK, Ueno-Kudo H, Asahara H. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–85. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Felsenfeld G. Chromatin unfolds. Cell. 1996;86:13–9. doi: 10.1016/s0092-8674(00)80073-2. [DOI] [PubMed] [Google Scholar]
- 23.Paranjape SM, Kamakaka RT, Kadonaga JT. Role of chromatin structure in the regulation of transcription by RNA polymerase II. Annu Rev Biochem. 1994;63:265–97. doi: 10.1146/annurev.bi.63.070194.001405. [DOI] [PubMed] [Google Scholar]
- 24.Culley KL, Hui W, Barter MJ, Davidson RK, Swingler TE, Destrument AP, Scott JL, Donell ST, Fenwick S, Rowan AD, Young DA, Clark IM. Class I histone deacetylase inhibition modulates metalloproteinase expression and blocks cytokine-induced cartilage degradation. Arthritis Rheumatol. 2013;65:1822–30. doi: 10.1002/art.37965. [DOI] [PubMed] [Google Scholar]
- 25.Joosten LA, Leoni F, Meghji S, Mascagni P. Inhibition of HDAC activity by ITF2357 ameliorates joint inflammation and prevents cartilage and bone destruction in experimental arthritis. Mol Med. 2011;17:391–6. doi: 10.2119/molmed.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong HM, Ding QH, Chen WP, Luo RB. Vorinostat, a HDAC inhibitor, showed anti-osteoarthritic activities through inhibition of iNOS and MMP expression, p38 and ERK phosphorylation and blocking NF-kappaB nuclear translocation. Int Immunopharmacol. 2013;17:329–35. doi: 10.1016/j.intimp.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 27.Makki MS, Haseeb A, Haqqi TM. MicroRNA-9 Promotion of Interleukin-6 Expression by Inhibiting Monocyte Chemoattractant Protein-Induced Protein 1 Expression in Interleukin-1beta-Stimulated Human Chondrocytes. Arthritis Rheumatol. 2015;67:2117–28. doi: 10.1002/art.39173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haseeb A, Makki MS, Haqqi TM. Modulation of ten-eleven translocation 1 (TET1), Isocitrate Dehydrogenase (IDH) expression, alpha-Ketoglutarate (alpha-KG), and DNA hydroxymethylation levels by interleukin-1beta in primary human chondrocytes. J Biol Chem. 2014;289:6877–85. doi: 10.1074/jbc.M113.512269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Makki MS, Heinzel T, Englert C. TSA downregulates Wilms tumor gene 1 (Wt1) expression at multiple levels. Nucleic Acids Res. 2008;36:4067–78. doi: 10.1093/nar/gkn356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwei KA, Finch JS, Thompson EJ, Bowden GT. Transcriptional repression of catalase in mouse skin tumor progression. Neoplasia. 2004;6:440–8. doi: 10.1593/neo.04127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen WP, Bao JP, Hu PF, Feng J, Wu LD. Alleviation of osteoarthritis by Trichostatin A, a histone deacetylase inhibitor, in experimental osteoarthritis. Mol Biol Rep. 2010;37:3967–72. doi: 10.1007/s11033-010-0055-9. [DOI] [PubMed] [Google Scholar]
- 32.Cai D, Yin S, Yang J, Jiang Q, Cao W. Histone deacetylase inhibition activates Nrf2 and protects against osteoarthritis. Arthritis Res Ther. 2015;17:269. doi: 10.1186/s13075-015-0774-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Messeguer X, Escudero R, Farre D, Nunez O, Martinez J, Alba MM. PROMO: detection of known transcription regulatory elements using species-tailored searches. Bioinformatics. 2002;18:333–4. doi: 10.1093/bioinformatics/18.2.333. [DOI] [PubMed] [Google Scholar]
- 34.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813:878–88. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 35.Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990;144:499–505. [PubMed] [Google Scholar]
- 36.Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine. 2010;51:178–83. doi: 10.1016/j.cyto.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 37.Rowan AD, Koshy PJ, Shingleton WD, Degnan BA, Heath JK, Vernallis AB, Spaull JR, Life PF, Hudson K, Cawston TE. Synergistic effects of glycoprotein 130 binding cytokines in combination with interleukin-1 on cartilage collagen breakdown. Arthritis Rheumatol. 2001;44:1620–32. doi: 10.1002/1529-0131(200107)44:7<1620::AID-ART285>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann Rheum Dis. 2012;71:424–31. doi: 10.1136/ard.2011.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Falkenberg KJ, Johnstone RW. Histone deacetylases and their inhibitors in cancer, neurological diseases and immune disorders. Nat Rev Drug Discov. 2014;13:673–91. doi: 10.1038/nrd4360. [DOI] [PubMed] [Google Scholar]
- 40.Grabiec AM, Krausz S, de Jager W, Burakowski T, Groot D, Sanders ME, et al. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J Immunol. 2010;184:2718–28. doi: 10.4049/jimmunol.0901467. [DOI] [PubMed] [Google Scholar]
- 41.Nasu Y, Nishida K, Miyazawa S, Komiyama T, Kadota Y, Abe N, Yoshida A, Hirohata S, Ohtsuka A, Ozaki T. Trichostatin A, a histone deacetylase inhibitor, suppresses synovial inflammation and subsequent cartilage destruction in a collagen antibody-induced arthritis mouse model. Osteoarthritis Cartilage. 2008;16:723–32. doi: 10.1016/j.joca.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 42.Iwasaki H, Takeuchi O, Teraguchi S, Matsushita K, Uehata T, Kuniyoshi K, Satoh T, Saitoh T, Matsushita M, Standley DM, Akira S. The IkappaB kinase complex regulates the stability of cytokine-encoding mRNA induced by TLR-IL-1R by controlling degradation of regnase-1. Nat Immunol. 2011;12:1167–75. doi: 10.1038/ni.2137. [DOI] [PubMed] [Google Scholar]
- 43.Nerlov C. The C/EBP family of transcription factors: a paradigm for interaction between gene expression and proliferation control. Trends Cell Biol. 2007;17:318–24. doi: 10.1016/j.tcb.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Ramji DP, Foka P. CCAAT/enhancer-binding proteins: structure, function and regulation. Biochem J. 2002;365:561–75. doi: 10.1042/BJ20020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poli V. The role of C/EBP isoforms in the control of inflammatory and native immunity functions. J Biol Chem. 1998;273:29279–82. doi: 10.1074/jbc.273.45.29279. [DOI] [PubMed] [Google Scholar]
- 46.Hirata M, Kugimiya F, Fukai A, Saito T, Yano F, Ikeda T, Mabuchi A, Sapkota BR, Akune T, Nishida N, Yoshimura N, Nakagawa T, Tokunaga K, Nakamura K, Chung UI, Kawaguchi H. C/EBPbeta and RUNX2 cooperate to degrade cartilage with MMP-13 as the target and HIF-2alpha as the inducer in chondrocytes. Hum Mol Genet. 2012;21:1111–23. doi: 10.1093/hmg/ddr540. [DOI] [PubMed] [Google Scholar]
- 47.Tsuchimochi K, Otero M, Dragomir CL, Plumb DA, Zerbini LF, Libermann TA, Marcu KB, Komiya S, Ijiri K, Goldring MB. GADD45beta enhances Col10a1 transcription via the MTK1/MKK3/6/p38 axis and activation of C/EBPbeta-TAD4 in terminally differentiating chondrocytes. J Biol Chem. 2010;285:8395–407. doi: 10.1074/jbc.M109.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirata M, Kugimiya F, Fukai A, Ohba S, Kawamura N, Ogasawara T, Kawasaki Y, Saito T, Yano F, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. C/EBPbeta Promotes transition from proliferation to hypertrophic differentiation of chondrocytes through transactivation of p57. PloS one. 2009;4:e4543. doi: 10.1371/journal.pone.0004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ushijima T, Okazaki K, Tsushima H, Iwamoto Y. CCAAT/enhancer-binding protein beta regulates the repression of type II collagen expression during the differentiation from proliferative to hypertrophic chondrocytes. J Biol Chem. 2014;289:2852–63. doi: 10.1074/jbc.M113.492843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vidal NOA, Ekberg S, Enerback, Lindahl A, Ohlsson C. The CCAAT/enhancer-binding protein-α is expressed in the germinal layer of the growth plate: colocalisation with the growth hormone receptor. J Endocrinol. 1997;155:433–441. doi: 10.1677/joe.0.1550433. [DOI] [PubMed] [Google Scholar]
- 51.Okuma T, Hirata M, Yano F, Mori D, Kawaguchi H, Chung UI, Tanaka S, Saito T. Regulation of mouse chondrocyte differentiation by CCAAT/enhancer-binding proteins. Biomed Res. 2015;36:21–9. doi: 10.2220/biomedres.36.21. [DOI] [PubMed] [Google Scholar]
- 52.Di Stefano B, Collombet S, Jakobsen JS, Wierer M, Sardina JL, Lackner A, Stadhouders R, Segura-Morales C, Francesconi M, Limone F, Mann M, Porse B, Thieffry D, Graf T. C/EBPα creates elite cells for iPSC reprogramming by upregulating Klf4 and increasing the levels of Lsd1 and Brd4. Nat Cell Biol. 2016;18:371–81. doi: 10.1038/ncb3326. [DOI] [PubMed] [Google Scholar]