Abstract
Demyelination in multiple sclerosis (MS) leads to significant, progressive axonal and neuronal degeneration. Currently existing immunosuppressive and immunomodulatory therapies alleviate MS symptoms and slow, but fail to prevent or reverse, disease progression. Restoration of damaged myelin sheath by replenishment of mature oligodendrocytes (OLs) should not only restore saltatory axon conduction, but also provide a major boost to axon survival. Our previous work has shown that therapeutic treatment with the modestly selective generic estrogen receptor (ER) β agonist diarylpropionitrile (DPN) confers functional neuroprotection in a chronic experimental autoimmune encephalomyelitis (EAE) mouse model of MS by stimulating endogenous myelination. Recently, we found that the more potent, selective ERβ agonist indazole-chloride (Ind-Cl) improves clinical disease and motor performance. Importantly, electrophysiological measures revealed improved corpus callosal conduction and reduced axon refractoriness. This Ind-Cl treatment-induced functional remyelination was attributable to increased OL progenitor cell (OPC) and mature OL numbers. At the intracellular signaling level, transition of early to late OPCs requires Erk1/2 signaling, and transition of immature to mature OLs requires mTOR signaling; thus, the PI3K/Akt/mTOR pathway plays a major role in the late stages of OL differentiation and myelination. Indeed, therapeutic treatment of EAE mice with various ERβ agonists results in increased brain-derived neurotrophic factor (BDNF) and phosphorylated (p) Akt and p-mTOR levels. It is notable that while DPN’s neuroprotective effects occur in the presence of peripheral and central inflammation, Ind-Cl is directly neuroprotective, as demonstrated by remyelination effects in the cuprizone-induced demyelination model, as well as anti-inflammatory. Elucidating the mechanisms by which ER agonists and other directly remyelinating agents modulate endogenous OPC and OL regulatory signaling is critical to the development of effective remyelinating drugs. The discovery of signaling targets to induce functional remyelination will valuably contribute to the treatment of demyelinating neurological diseases, including MS, stroke, and traumatic brain and spinal cord injury.
Keywords: estrogen receptor ligands, multiple sclerosis, demyelination, remyelination, experimental autoimmune encephalomyelitis, axon degeneration
Introduction
Multiple sclerosis (MS) is a neurodegenerative, inflammatory demyelinating disease of the central nervous system (CNS) which affects 2 to 2.5 million people worldwide[1] [2]. MS causes a variety of neurological deficits that severely impact the patient’s quality of life (e.g., loss of vision, sensory deficits, ataxia, paralysis, brainstem dysfunction, dementia, psychiatric disorders [3]. MS is considered an autoimmune disease, a systemic or tissue-specific disorder in which the immune system attacks the body’s own tissue. Silencing autoreactive T- and B-lymphocytes normally helps to prevent development of autoimmune disease. [4]. Myelin-specific CD8+ T cells are the primary mediators of MS, which is characterized by multiple lesions called “plaques” [5,6]. CD8+ T lymphocytes, B lymphocytes, microglia, macrophages, and reactive astrocytes contribute to the cellular composition of these inflammatory lesions, along with oligodendrocyte (OL) death and demyelinated axons [1]. Nonetheless, emerging evidence has begun to implicate autoreactive CD4+ T cells in MS pathogenesis[4], and indeed, the HLA-DR gene encoding MHC-II has been implicated as an MS susceptibility site [5]. Continued lesion formation leads to physical disability and cognitive decline [1].
Most commonly, MS presents in the relapse-remitting form (RRMS), which is characterized by acute episodes of disability followed by recovery. In this disease stage, lesions may exhibit remyelination and continued presence of immune cells [1]. As patients age and experience multiple relapses with decreasing remittance, the disease enters the progressive stage [7]. Heterogeneity in MS pathology makes it difficult to classify progressive MS lesions, as both CD8+ T cells and CD68+ microglia are observed adjacent to damaged neurons participating in neurodegenerative processes [8], whereas some chronically demyelinating lesions distinctly lack immune cell infiltration or activated glial cells [9]. As neurodegeneration ensues, patients develop permanent motor and cognitive impairments.
MS is a multifaceted disease with an unknown etiology and no effective cure. Researchers have limited access to active MS tissue samples, and manipulation of experimental circumstances is restricted in human studies compared to studies in animal models, although new imaging modalities have yielded helpful insights in patients, especially the white matter and ophthalmologic manifestations. For these reasons, animal models such as experimental autoimmune encephalomyelitis (EAE) are needed to clarify the underlying immune-pathological mechanisms and test novel therapeutic and reparative approaches [10]. EAE, is the most common inducible animal model of MS. It recapitulates inflammation, demyelination, and neurodegeneration components of MS disease. EAE is a CD4+ T cell–mediated autoimmune disease, whereas human MS is a macrophage, B cells and CD8+ T-cell mediated disease. EAE induction requires immunization with myelin proteins or peptides. EAE model has improved our overall understanding of CNS inflammation, immune surveillance and immune-mediated tissue injury. Furthermore, therapeutic studies using the EAE model have led to the development of approved MS treatments [10].
In this review, we hope to illustrate the need for combined immunomodulatory and neuroprotective therapies in MS. We begin with a discussion of pathophysiology, framing a central problem in MS therapy: the differentiation block of OPCs in the face of ongoing inflammation that contributes to remyelination failure and disease progression. Next, we explore the contribution of endogenous estrogens on the disease process; the preclinical advancements in mouse models of MS by targeting the estrogen receptor (ER) with ER-selective ligands; the signaling pathways affected by ER agonists; and future directions in the field.
Axon Myelination
Myelin sheath, the insulating layer surrounding the axon, is required for efficient function in the vertebrate nervous system. It is responsible for maximizing the conduction velocity of action potentials [6]. In the central nervous system (CNS), OLs, a terminally differentiated, nonproliferative population of cells are responsible for myelin production. The number of differentiated OLs is dependent on the extent of proliferation of the OL precursor cell (OPC). During early embryonic development, OPCs are generated in the ventral neuroepithelial motor neuron progenitor domain of the neural tube [7, 8]. During late embryonic and early post-natal development, the dorsal spinal cord and hindbrain/telencephalon are responsible for OPC production [9, 10]. OPCs are proliferative and motile cells expressing GT3 ganglioside A2B5, platelet-derived growth factor receptor alpha (PDGFRα), and the proteoglycan NG2 [11]. OPC specification is regulated by the basic helix–loop–helix (bHLH) transcription factors Olig1, Olig2, mammalian achate schute homolog 1 (Mash1), and the zinc finger transcription factor myelin transcription factor 1 (MyT1) [12–14]. Proliferative OPCs migrate into developing white matter, exit the cell cycle, undergo differentiation, express a subset of myelin-associated proteins, and transition to mature OLs [15]. Various agents, including insulin-like growth factor (IGF)-1, ciliary neurotrophic factor (CNTF), and thyroid hormone (T3), are involved in promoting OPC differentiation to OLs along the oligodendroglial lineage [16–18]. Myelination consists of OL plasma membrane loops tightly wound concentrically around the axon. One OL can produce up to forty myelin segments on multiple axons [15]. Myelin membrane maintenance occurs throughout childhood and young adulthood leading to maturation of the complex neural circuitry. Classically myelination is thought to complete when the frontal lobes finish myelination in the fourth decade of human life [19, 20].
Demyelination and Remyelination
Demyelination is the pathological process in which myelin sheaths surrounding axons are lost. CNS “primary” demyelination is usually the consequence of OL loss, whereas “secondary” demyelination (or Wallerian degeneration) is a consequence of primary axonal loss [21]. Demyelination impairs neuronal function. It destroys reciprocal communication between axons and OLs, and the acute loss of a myelin internode is associated with conduction block. In MS, OL loss and demyelination are associated with disruption of the paranodal region, progressive axonal degeneration, and neurological decline [1]. An immediate adaptive response of Na+ channel redistribution along demyelinated axons allows for some non-saltatory conduction [21]. Recovery from MS relapses (remittances) can reflect the resolution of exaggerated inflammatory response, consequent sparing of neural cells, and myelin repair (remyelination), and leading to the functional reorganization of nodal components and recovery of some axon function.
Remyelination involves the generation of new mature OLs and is the default spontaneous process by which demyelinated axons undergo ensheathment by new myelin sheaths, leading to functional recovery. During remyelination in the adult CNS, OLs are derived from a population of adult CNS stem/progenitor cells referred to as adult OPCs [22, 23]. These multiprocessed adult OPCs are found in both white and grey matter throughout the CNS, at a density 5–8% of the cell population [24]. Adult OPCs arise from the subventricular zone (SVZ), either from progenitors that contribute to the rostral migratory stream or from glial-fibrillary acidic protein (GFAP)-expressing stem cells [25]. These OPCs are generally quiescent until activated by homeostatic insult, such as OL death and axon demyelination. Adult OPC activation is likely secondary to astrocytic and microglial activation [26], and growth factors upregulated during remyelination induce OPC proliferation, migration, and differentiation into mature, myelinating OLs [27].
In a healthy CNS, the regenerative process that underlies endogenous remyelination is initiated and proceeds to completion naturally in response to minor demyelinating insults such as infectious, metabolic, immune, and other demyelinating perturbations [28]. In diseases such as MS, demyelination, along with substantial axonal and neuronal loss, forms a major part of the pathology. A limited number of MS patients exhibit spontaneous remyelination in ~95% of lesions and OPC numbers are increased in actively demyelinating MS plaques [29]. However, remyelination eventually fails in MS and the disease inches towards the progressive phase. Theoretically, remyelination could fail because of a deficiency in precursor cells, failure of progenitor cell recruitment, and/or failure of OPC differentiation and maturation [30]. Other remyelination failure hypotheses include changes in environmental inputs or intrinsic signals regulating OPC function [21]. Each of the above hypotheses is possible to some degree. First, over time OPCs are depleted in most progressive MS patients, leading to insufficient remyelination as the disease progresses [22, 29, 31]. A failure of OPC recruitment involving proliferation, migration, and repopulation of demyelinated areas also contributes to remyelination failure [32]. Lastly, a failure of OL differentiation and maturation in areas of demyelination indicates that this stage of remyelination is most vulnerable to failure in MS [28, 32, 33].
Stimulating Functional Remyelination
Understanding and stimulating remyelination in human MS patients presents a challenge. While the dynamic nature of remyelination may be assessed murine models in vivo, it is difficult to investigate this complex process ex vivo (e.g., in post-mortem tissue) [34, 35]. Thus, a thorough understanding of therapeutics-induced remyelination in the context of MS requires comparative analyses of animal models and human tissue.
To attain efficient remyelination, it may be necessary to investigate genes, transcription factors, and signaling molecules controlling OPC differentiation during development, as they may play similar roles in activated adult OPCs. Consistent with this, expression of transcription factor Olig2 and homeodomain transcription factor Nkx2.2 in OPCs is a genetic switch required for OPC differentiation [36]. The expression of these factors converges in OPCs during development and is upregulated in response to demyelination [37]. In addition, growth factors IGF, PDGF, and BFGF can act individually as mitogens for OPCs in vitro. A number of extracellular signals that regulate a series of transcription factors that promote OPC differentiation to myelinating OLs have been identified ([38], reviewed in [15]). The mTOR (mammalian target of rapamycin) pathway, which is downstream of PI3K (phosphoinositide 3-kinase)/Akt, promotes OL differentiation and myelination [39–42]. In addition, ERK1/2-MAPK (mitogen-activated protein kinase) [43] regulates myelin thickness, independent of OL differentiation, and myelination initiation [44]. The other signaling pathways and molecules behind impaired remyelination in animal models include involvement of the Notch1, LINGO1, wnt/β catenin, and hyaluronan/TLR2 pathways (reviews [45] [46]).
Most of these relevant molecules, many of which were identified in developmental myelination studies, are detected in MS. OPCs that express PDGFRα can be abundant in MS lesions [47] and PDGF and FGF-2 ligand expression increases in demyelinated lesions [48]. An integral component of the environment surrounding OPCs is neurotransmitters that could, potentially, regulate integrin-mediated functions such as survival, proliferation, and migration [49]. Several other factors have been shown to either promote or inhibit remyelination. Endogenous remyelination is promoted by cytokines tumor necrosis factor-α (TNFα) [50] and interleukin 1β [51], chemokines CXCL-12 and CXCR4 [52], and growth factors PDGF, BFGF, and IGF-1 [53–58]. In contrast, other molecules such as the axon-derived polysialylated form of the neural cell-adhesion molecule (NCAM) [59], contactin-a non-canonical Notch1 ligand [60], wnt/β catenin [61], the Ig domain–containing Nogo receptor–interacting protein LINGO-1[62], myelin debris [63], and hyalourinadase [64] inhibit myelination and contribute to remyelination failure.
Current therapeutic strategies for MS have focused on attenuating the immune response. Immunomodulators [Interferon beta-1b (Betaseron, Extavia), Dalfampridine (Ampyra), Interferon beta-1a (Avonex, Rebif), Alemtuzumab (Lemtrada), Peginterferon beta-1a (Plegridy), Natalizumab (Tysabri), Glatiramer acetate (Copaxone), Fingolimod (Gilenya), Dimethyl fumarate (Tecfidera)] and corticosteroids [Methylprednisolone (Solu-Medrol, Depo-Medrol), Dexamethasone (Baycadron, Dexamethasone Intensol), and Prednisone (Sterapred[65] are indicated for treatment of RRMS patients. These compounds slow the accumulation of physical disability and decrease the frequency of clinical exacerbations; however, they do not reverse existing damage. Additionally, the utility of anti-inflammatory therapies is limited because progressive MS lesions do not exhibit a strong inflammatory component. Accumulating data regarding MS lesions and the influence of regenerative medicine have begun to shift the field’s understanding of the role inflammation in MS. Originally, a link between the presence of immune cells and lesions was observed [66]. Over time, however, a more nuanced perspective has revealed a link between immune activation and successful remyelination [21]. Inflammation in MS has been reframed into a dichotomous role: it is necessary to resolve the destruction it initially causes. Whatever the order of these pathological events, it is clear that preventing inflammation, demyelination, and neurodegeneration, or enhancing remyelination and neuroprotection, are all potential targets for therapies that reduce disease and disability in MS. Ideal drug therapies will need to address both myelin sheath protection and subsequent repair via remyelination modulation.
Approved MS treatments, as well as those currently in the pipeline, target some aspects of the above-referenced signaling directly or indirectly to initiate transient recovery. Whether some of these treatments also act on the CNS to directly promote myelin repair remains debated. One promising remyelinating drug is the anti-LINGO antibody [67]. Biogen’s BIIB033, a human aglycosyl IgG1 monoclonal antibody (mAb) that binds LINGO-1 with high affinity and specificity, was developed as an investigational drug aimed at inducing remyelination and axonal protection and/or repair in MS patients. This antibody yielded positive results in a phase II trial for acute optic neuritis (AON), a relatively uncommon disease (and condition that can precede MS diagnosis) [68, 69]. A recent study screened 727 drugs with a history of safe use in clinical trials from the US National Institutes of Health (NIH) Clinical Collection I and II libraries for modulation of OPC maturation into myelinating OLs [70]. Two steroids, miconazole and clobetasol, were effective in promoting myelination and decreasing clinical symptoms in mouse models of MS [70].
Estrogens
Experimental evidence supporting neuroprotective actions by steroid hormone, estrogen has accumulated over the last two decades. For example, local synthesis of these molecules in the CNS may prevent or reduce neurodegeneration [71]. Defensive steroid hormone actions include stabilizing the blood-brain-barrier (BBB), alleviating brain edema, dampening pro-inflammatory processes, activating anti-apoptotic molecules, stimulating survival-promoting factors, counteracting oxidative stress, promoting respiratory chain function, and reducing glutamate excitotoxicity [72]. Genomic and non-genomic steroid signaling pathways appear to mediate defensive steroid hormone actions leading to CNS neuroprotection [73]. The three major estrogens are estradiol (also referred to as E2 or 17β-estradiol), estrone (E1), and estriol (E3). Estrogens regulate transcription by binding to nuclear estrogen receptors (ER), ERα and ERβ, which exhibit distinct transcriptional properties[74], whereas, plasma membrane-associated ERs mediate the non-genomic signaling pathway[75] (Figure 1). Both ERα and ERβ are localized in reproductive tissues, as well as non-reproductive tissues including cells of the immune system, cardiovascular system, CNS and bone. Within the immune system, ERs are present in B lymphocytes, T lymphocytes, monocytes, and NK cells [76].
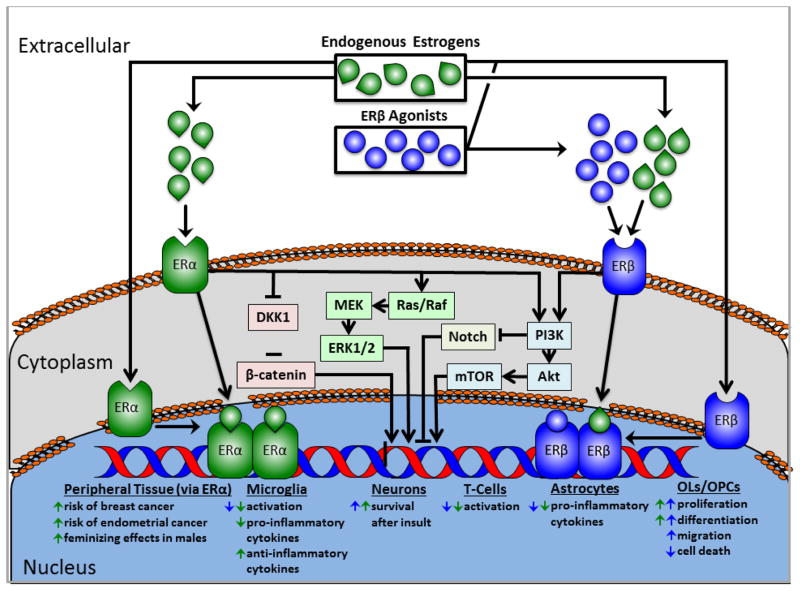
Figure 1.
Schematized general estrogen signaling pathway. Endogenous estrogens and estrogen receptor (ER) agonists can activate cell and nuclear membrane-bound ERs. Binding induces dimerization and translocation of ERs to the nucleus, where they exert effects on transcription. Binding can also affect intracellular signaling pathways, including PI3K/Akt/mTOR, Wnt, and Notch, ultimately influencing gene expression. ERα and β each activate the PI3K/Akt/mTOR pathway, and activation of PI3K inhibits Notch. ERα activation inhibits dickkopf WNT signaling pathway inhibitor 1 (DKK1), resulting in β-catenin stabilization. The cell type-specific molecular mechanisms mediating the effects listed here have yet to be parsed out for neural cells.
In human and animal experimental models, estrogens have been shown to regulate both innate and adaptive immunity [77]. Inflammation resulting from immune aggression during the innate or adaptive immune response involves the release of pro-inflammatory cytokines. Estrogen are immunomodulatory and may exert immunosuppressive and immunostimulatory effects [78]and induce CNS neuroprotection. Here, we will focus on the role of estrogens in CNS neuroprotection.
Estrogens and MS
The use of estrogens as a treatment for MS is based on the remarkable effects of pregnancy and the post-partum period on disease activity in patients with MS and other autoimmune diseases, including rheumatoid arthritis and psoriasis[79] [80, 81]. These patients experience clinical improvement during pregnancy followed by a temporary ‘rebound’ exacerbation postpartum. Increased levels of a variety of pregnancy-related factors including estrogens (i.e., estradiol and estriol) are detected in maternal blood during pregnancy and many, including cortisol, progesterone, vitamin D, early pregnancy factor, and α-Fetoprotein, have been shown to be immunomodulatory and possess potential neuroprotective properties[82]. Estrogens downregulate the T cell-mediated adaptive immune response, simultaneously activate the innate immune response that tolerates, supports, and regulates fetal development, and confer neuroprotection and reduce relapses in pregnant women with MS[83].
There exists a temporal correlation between increased estrogen levels in the last trimester of pregnancy and MS remission, and between decreased estrogen levels post-partum and MS relapse [83]. Thus, estrogens represent therapeutic candidates for MS. Preclinical studies using mouse models of MS reveal an attenuated disease course in response to estriol administration at doses equivalent to late pregnancy levels[84]. While clinical observations of pregnant MS patients are correlative, pre-clinical studies support a causative link between increased estrogen levels and neuroprotection [85, 86]. While estrogens may play a role in MS remission during late pregnancy, this does not preclude additional or synergistic roles for other pregnancy factors [79]. In a small single-arm study of people with MS, estriol reduced gadolinium-enhancing lesions and was favorably immunomodulatory [87]. In a recently completed phase II trial using estriol in combination with glatiramer acetate to treat female RRMS patients showed a small decrease in relapse rate compared to placebo with glatiramer acetate [88]. However, the use of estrogens as a therapy must be carefully considered, as these compounds are highly reactive in many tissues. Estrogen therapies present several potential side effects, including increased risk of breast and endometrial cancers in females and feminizing effects in males. Recent synthesis of a small-molecule bioprecursor prodrug, 10β, 17β-dihydroxyestra-1, 4-dien-3-one (DHED), which converts to estradiol in the brain after systemic administration but remains inert in the rest of the body, could bypass negative peripheral side effects associated with estrogens [89]. Interestingly, the carcinogenic effects of estrogens are reportedly mediated through ERα and not ERβ, which supports the concept of receptor subtype-specific treatments [90].
Estrogens and Oligodendrocytes
OLs and OPCs express nuclear and membrane ERα and ERβ mRNA and protein in in vitro and in vivo [91–95]. The classical estrogen-signaling pathway involves the binding and activation of ERα and ERβ receptors and subsequent transcriptional activation of appropriate target genes. Estrogen and various growth factors such as BDNF and IGF-1 activate similar signaling pathways that lead to OL differentiation and myelination [44, 71, 96]. Both estradiol and BDNF enhance OPC differentiation to OLs through the Trk-B receptor [97]. Reduced levels of BDNF, OPCs, and myelin proteins were observed in transgenic mice heterozygous for BDNF [71, 98]. The association of estrogens with IGF-1R/Trk-B through the PI3K/Akt/mTOR signaling pathway may illustrate the point of convergence between estrogen and IGF-1/BDNF to promote OPC proliferation, OL differentiation, and survival after demyelination [38, 71, 99]. Estrogen ligands that successfully activate these pathways to initiate OPC/OL survival, OL differentiation, and axon myelination would be most effective in prompting remyelination and neuroprotection.
ERβ Ligands - Similarities and Differences in Structure and Mechanism
Selective estrogen receptor modulators (SERMs) are ER ligands that show preferential binding affinity towards one receptor isotype over the other. SERMs may be extremely useful in estrogen therapies, as they allow for targeting of specific tissues or signaling pathways based on differences in receptor isotype activity and distribution within tissues. Both ERα and ERβ are expressed in the immune system and CNS [74]. Clinical protection from EAE by estradiol is dependent on signaling through ERα [100, 101]. Interestingly, the anti-inflammatory effects of estrogens are mediated by ERα [74]. More specifically, treatment with the ERα–selective ligand propylpyrazoletriol (PPT) was sufficient to ameliorate EAE and induce favorable changes in autoantigen-specific cytokine production in the peripheral immune system, and decreased CNS peripheral immune infiltration in EAE [102]. Roles for ERβ are mixed, as different ERβ-specific ligands induce multiple therapeutic effects including immunomodulation and direct neuroprotection [102]; [103], as will be discussed below.
Several synthetic and natural ERβ-selective compounds with differential ERβ affinities and selectivities have been identified [104–106];[107];[108]. One class of ERβ-selective agonists, represented by WAY-202041 (ERB-041) and WAY-200070, display >200-fold selectivity for ERβ over ERα. IC50 values are 5 and 1216 nM for human ERβ and ERα, 3.1 and 620 nM for rat ERβ and ERα, and 3.7 and 750 nM for mouse ERβ and ERα, respectively [109]. ERB-041 regulates hippocampal synaptic plasticity and memory improvement in gonadectomized mice [110] and exerts an anti-inflammatory effect in a lipopolysaccharide (LPS) model [54]. Clinically-relevant applications have included inflammatory bowel disease, rheumatoid arthritis, endometriosis, and sepsis [111, 112], but ERB-041 failed to improve EAE [113]. In a recent clinical trial, it failed to suppress rheumatoid arthritis [114]. Meanwhile, WAY-200070 has also been shown to improve hippocampal synaptic plasticity and memory improvements [115] [110]. Finally, DPN used in published studies from our lab, represents a third class of ERβ-selective compounds. DPN was tested along with the ERα–selective ligand propylpyrazoletriol (PPT) in myelin oligodendrocyte glycoprotein (MOG35–55) EAE-induced C57BL/6 mice [102]. This produces a chronic disease course similar to progressive MS; thus, it represents a rigorous model to test potential MS therapeutics. Prophylactic treatment (i.e., treatment start prior to clinical disease onset) with PPT was sufficient to prevent/blunt EAE onset and induce favorable changes in autoantigen-specific cytokine production in the peripheral immune system [101]. While prophylactic and therapeutic (i.e., treatment start after clinical disease onset) treatment of MOG35–55-induced EAE C57BL/6 mice with DPN had no effect on peripheral cytokine production or CNS infiltration, it increased axon myelination and corpus callosal axon conduction and improved motor function [94, 100].
Similar biological activities for these three classes of ERβ-selective compounds have demonstrated that while some genes are commonly regulated by these agonists, others are differentially regulated by these agonists and estradiol [106]. Very recently, another class of highly selective ERβ ligand with a halogen-substituted phenyl-2H-indazole core, indazole-Cl (Ind-Cl), was shown to suppress inflammatory responses of microglia and astrocytes in vitro [107, 116] and attenuate EAE clinical symptoms[116]. Though Ind-Cl’s immunomodlulatory effect is distinct from DPN’s lack of effect on the immune response, treatment with either ligand decreases EAE clinical symptoms via direct neuroprotection. Below, we discuss a few major ERβ ligands that show promise in the chronic EAE mouse model and an overview of their effects can be found in Figure 2.
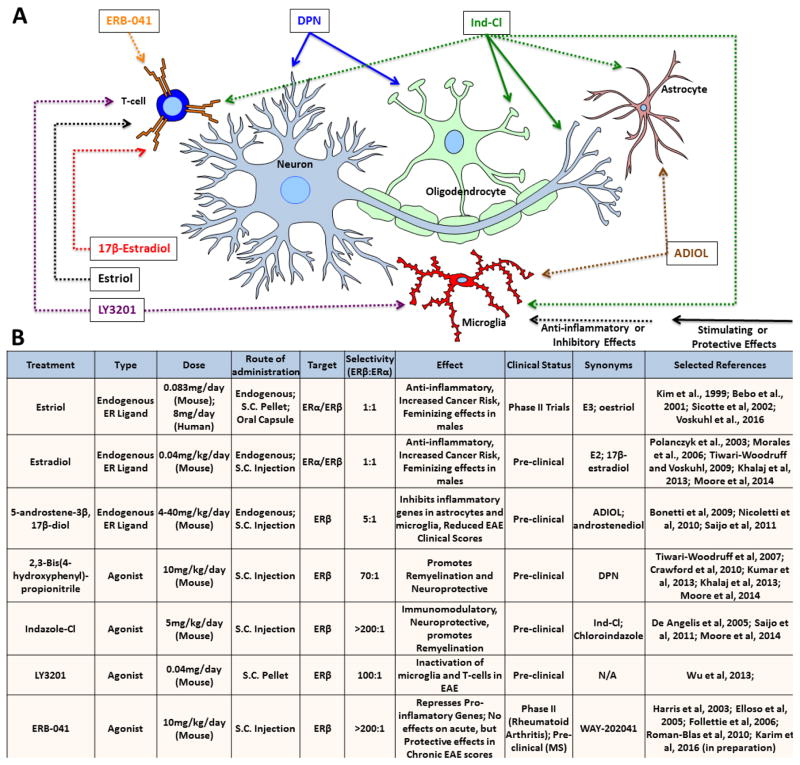
Figure 2.
Therapeutic effects of endogenous estrogens and selective estrogen receptor (ER) agonists on various CNS cell types.
(A) ERs are ubiquitously expressed, and ER ligands have differential effects on different cell types. This diagram details experimentally-determined effects of each ligand in various pathological models (hence the depiction of T-cell CNS infiltration). Solid arrows: stimulating/protective effects; dashed arrows: anti-inflammatory/inhibitory effects.
(B) Dosage, route of administration, selectivity, effects, and clinical status of various ER ligands.
1. DPN
DPN is a generic ERβ ligand with 70-fold higher relative binding affinity and 170-fold higher relative potency for ERβ over ERα in transcription assays [117]. DPN exists as a racemic mixture of two enantiomers, (R)-DPN and (S)-DPN. Both enantiomers show high affinity and potency for ERβ over ERα (80–300-fold). The enantio-selectivity is modest (~3–4-fold), with the (R)-enantiomer possessing higher affinity and potency. While ERβ is effectively stimulated by (R,S)-, (R)-, or (S)-DPN, (R)-DPN may be favored for biological studies of ERβ function [108, 118].
When administered prophylactically or beginning at peak clinical disease, DPN (racemic) reduces EAE clinical symptoms in MOG35–55-induced C57BL/6 mice [94, 119]. Unlike reports using other estrogens, such as estradiol, therapeutic improvement in this chronic EAE model is not immediate [101]. Improvement was observed 20 days after initiation of prophylactic treatment [94, 120]. Further, when treatment was begun 21 or 34 days post-EAE induction, improvement was observed after one and one and a half weeks, respectively [94]. DPN successfully attenuated clinical disease and improved motor function in EAE mice with no effect on the immune response, providing evidence of direct neuroprotection. Furthermore, DPN-induced EAE clinical improvements were associated with increased mature OL numbers, increased myelin thickness, amelioration of corpus callosal axon conduction deficit, and improved axon transport, all in the presence of an active immune environment [100, 119]. These results are of great interest in light of accumulating evidence connecting active immune response to successful remyelination in MS lesions.
Which cell type(s) mediate DPN’s direct neuroprotective effects? As the myelinating cells of the CNS, OLs are of particular interest in MS and EAE. Using Cre recombinase technology, we generated conditional knock-out mice lacking ERβ in all OL lineage cells (ERβ-Olig2 cKO). DPN treatment in MOG35–55 EAE ERβ-Olig2 cKO mice failed to induce many of the key improvements observed in DPN-treated littermate controls [93]. ERβ deletion from astrocytes or neurons, however, did not affect DPN-induced EAE improvements [121]. This suggests that much of DPN-induced EAE improvement is mediated through direct action on ERβ in OL lineage cells [93].
As for DPN-induced downstream signaling in EAE, DPN treatment increases BDNF levels and activates the PI3/AKT/mTOR pathway, which is essential for OL differentiation and CNS myelination (discussed above) [94]. This suggests that DPN and DPN-like ERβ agonists may be capable of overcoming the OL differentiation block in chronically demyelinated MS lesions, which represents a major goal for progressive MS therapeutics.
DPN is of particular interest in the study of MS and EAE because it does not show significant immune modulation [102, 103]. Decoupling the anti-inflammatory component of ERβ action allows researchers to investigate direct effects of ERβ ligands on remyelination and disease treatment. Though this is useful in the pre-clinical setting, DPN’s low specificity and high hydrophobicity may lead to non-specific side effects with prolonged or high dosage use [120]. As a result, there is a need to identify ERβ ligands that reduce MS symptoms with greater potency and safety.
2. Indazole-Cl
Ind-Cl is a small molecule, potent ERβ selective agonist based on a halogen-substituted phenyl-2H-indazole core that[122] displays >200-fold selectivity for ERβ over ERα [122]. It is in pre-clinical development and its promising pharmacokinetics are well-characterized in rodents [123].
In contrast to DPN, Ind-Cl is immunomodlulatory as well as directly neuroprotective [103]. Prophylactic and therapeutic Ind-Cl treatment in MOG35–55-induced EAE C57BL/6 mice reduced invading immune cell numbers and resident pro-inflammatory cell activation in the CNS [103]. Overall peripheral immune activation was decreased by Ind-Cl treatment, as quantified by reduced Th1 and Th17 cytokine production (including IFN-γ, IL-6, TNF-α, and IL-17) in isolated MOG35–55-stimulated splenocytes [103]. Ind-Cl and a similar molecule made with the halogen bromine, Indazole-Br (Ind-Br), have also been shown to radically alter the cytokine profile of human and murine microglia in vitro via transrepression, ultimately increasing anti-inflammatory and reducing pro-inflammatory cytokine production[116].
Several results from experiments in Ind-Cl-treated MOG35–55-induced chronic EAE mice point towards the induction of functional remyelination. These include increased myelin basic protein (MBP) levels and myelinating OL numbers in the spinal cord and corpus callosum. Additionally, treatment increased survival, proliferation, and migration of OPCs from the SVZ to CC lesions [103]. At the functional level, prophylactic and therapeutic Ind-Cl treatment improved CC axon conduction and rotarod motor activity in EAE mice.
Similar to DPN, Ind-Cl treatment in chronic EAE mice increased BDNF levels and activated the PI3/AKT/mTOR pathway [103]. In the chronic demyelination cuprizone model (nine weeks of continuous 0.2% cuprizone diet) followed by three weeks of remyelination (normal diet), Ind-Cl treatment increased axon remyelination and OL numbers [103]. Unlike EAE, the cuprizone model allows experimenters to isolate myelination events from primary inflammation. These findings suggest that Ind-Cl is capable of improving remyelination in both inflammatory and non-inflammatory settings. Thus, Ind-Cl represents a promising, more selective progressive MS treatment option, as it may overcome the differentiation block in chronically demyelinated lesions.
3. 5-androsten-3β, 17β-diol (ADIOL)
ADIOL is an endogenous ERβ ligand produced by enzymatic conversion of the steroid dehydroepiandrosterone (DHEA). There is a genetic link between ADIOL and MS because the enzyme that converts DHEA to ADIOL, 17β-hydoxysteroid dehydrogenase type 14 (HSD17B14), is located at a locus of human chromosome 19 that has been identified as an MS susceptible locus [124]. This compound acts via transrepression to activate ERβ to recruit a protein complex which inhibits inflammatory gene expression in microglia and astrocytes [116]. When administered at disease onset, this compound caused a transient dose-dependent decrease in clinical symptoms of proteolipid protein (PLP139-151)-induced EAE SJL/J female mice, a RRMS model [125]. While further studies are needed, the discovery of an endogenous ERβ ligand with a genetic connection to MS susceptibility adds weight to the pursuit of estrogen ligands to treat MS.
4. LY3201
LY3201 is an ERβ-selective ligand provided by Eli Lilly to Gustafsson and colleagues as a potential MS treatment candidate. A RRMS-like EAE model, generated using SJL/J mice immunized with proteolipid protein (PLP), was used to test LY3201 prophylactic treatment effects. LY3201 reduced EAE-induced mortality within the first two weeks of disease induction from ~50% to <10%. Within thirty days, it significantly attenuated EAE clinical disease [126]. Effects on OPCs, OLs, functional myelination, and a chronic EAE model were not assessed. LY3201 treatment reduced microglial activation and downregulated NF-κB activation and NF-κB-induced gene-inducible nitric oxide synthase (iNOS) in microglia and CD3 (+) T cells of PLP-induced EAE mice [126]. Whether immune modulation via microglia and T cells—and, thus, contribution to the regenerative cytokine environment encountered by OPCs and OLs—by LY3201 or LY3201-like ERβ ligands induces remyelination and repair remains to be tested.
5. ERB-041
ERB-041 is benzoxazole compound and is among the most selective ERβ ligands, with 200-fold selectivity for ERβ over ERα [127]. ERB-041 has been shown to improve several rodent models of inflammatory disease in vivo, including the HLA-B27 transgenic rat model of inflammatory bowel disease and adjuvant-induced arthritis in Lewis rats [127]. The ligand was administered orally and resulted in quantitative abrogation of sepsis and endometriosis [111, 127, 128]. We have published [103] and preliminary data revealing decreased MOG35–55-induced EAE clinical scores with both prophylactic and therapeutic ERB-041 treatment (administered subcutaneously). Additionally, preliminary in vivo data from our lab suggest that this ligand has the ability to modulate the peripheral immune response. Interestingly, while in vivo studies cited above indicate an immunomodulatory effect based on improved disease outcomes, the mechanisms of immune modulation appear to be at least partially distinct from Ind-Cl and similar to DPN in the inability to reduce iNOS production in LPS-stimulated microglia [54]. This suggests that the anti-inflammatory effects of ERB-041 are regulated through an alternate pathway and reinforces the view that anti-inflammatory effects of ERβ ligands are cell type-specific. In addition, this compound has been tested in a version of EAE that results in an acute, monophasic disease course but the results did not show a significant delay in disease onset or peak clinical scores[113]. Despite this, and due to the relatively short symptomatic period in that model, there is still a need for further study of this compound in either relapse-remitting or primary progressive EAE to test the later stage effects of this drug. ERB-041 has already gone through a Phase I trial and was deemed safe, it could be optimized and tested as potential MS therapeutic.
Summary and Future Directions
Historically, the argument for promoting axon remyelination is based on improved conduction efficiency via non-saltatory to saltatory conduction switch. Our current appreciation of axon loss as a major pathological correlate of progressive functional decline in demyelinating neurodegenerative diseases has created an even more compelling case to study remyelination. Promoting myelin repair represents a potentially highly effective means of long-term axon protection and repair. Therefore, a key therapeutic goal for MS is to remyelinate naked axons rapidly, thus repairing existing and preventing cumulative disability. Existing data like those discussed here and studies currently underway support ERβ ligands as potential remyelinating, neuroprotective agents that may be used alone or in combination with other therapeutics (e.g., approved immunomodulators) to attain functional recovery in MS.
Through chemical modification of naturally occurring estrogens and synthesis of compounds that mimic their neuroprotective effects, we may produce more selective compounds that provide the benefits of estrogens to the CNS while avoiding negative side effects. ERβ ligands represent exciting potential MS therapeutics. DPN and Ind-Cl-induced activation of PI3/Akt/mTOR signaling suggests that these signaling molecules may be capable of overcoming the differentiation block observed in chronic MS lesions. In addition, early experiments by our lab suggest that these ligands are also capable of ERK activation. The variety of mechanisms triggered by different ERβ ligands warrants continued investigation. Finally, the isolation of neuroprotective and immune effects of some ERβ ligands offers new possibilities for combination therapy, allowing for more fine-tuned and personalized treatment of highly variable MS.
Highlights.
Pregnancy is associated with remission in patients with multiple sclerosis (MS).
Estrogen receptor β agonists stimulate functional remyelination in MS mouse models.
Some estrogen receptor beta ligands activate PI3K/Akt/mTOR signaling pathways in oligodendrocytes to stimulate remyelination
ERβ agonists effects induce multiple therapeutic effects on the CNS and are devoid of negative side effects associated with ERα agonists
ERβ agonists represent promising potential novel therapeutics for MS.
Acknowledgments
This work was generously supported by NMSS RG 4853A3/2, NIH R01 NS081141-01A1, NIH R21NS075198 to STW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–93. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 2.Browne P, et al. Atlas of Multiple Sclerosis 2013: A growing global problem with widespread inequity. Neurology. 2014;83(11):1022–4. doi: 10.1212/WNL.0000000000000768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van der Valk P, De Groot CJ. Staging of multiple sclerosis (MS) lesions: pathology of the time frame of MS. Neuropathol Appl Neurobiol. 2000;26(1):2–10. doi: 10.1046/j.1365-2990.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 4.Hohlfeld R, et al. The search for the target antigens of multiple sclerosis, part 1: autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2015 doi: 10.1016/S1474-4422(15)00334-8. [DOI] [PubMed] [Google Scholar]
- 5.Hollenbach JA, Oksenberg JR. The immunogenetics of multiple sclerosis: A comprehensive review. J Autoimmun. 64:13–25. doi: 10.1016/j.jaut.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3(2):141–50. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- 7.Pringle NP, et al. Dorsal spinal cord neuroepithelium generates astrocytes but not oligodendrocytes. Neuron. 1998;20(5):883–93. doi: 10.1016/s0896-6273(00)80470-5. [DOI] [PubMed] [Google Scholar]
- 8.Noll E, Miller RH. Oligodendrocyte precursors originate at the ventral ventricular zone dorsal to the ventral midline region in the embryonic rat spinal cord. Development. 1993;118(2):563–73. doi: 10.1242/dev.118.2.563. [DOI] [PubMed] [Google Scholar]
- 9.Qi Y, Stapp D, Qiu M. Origin and molecular specification of oligodendrocytes in the telencephalon. Trends Neurosci. 2002;25(5):223–5. doi: 10.1016/s0166-2236(02)02145-8. [DOI] [PubMed] [Google Scholar]
- 10.Kessaris N, et al. Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci. 2006;9(2):173–9. doi: 10.1038/nn1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3(6):191–7. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- 12.Ffrench-Constant C, Raff MC. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319(6053):499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 13.Nakatani H, et al. Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci. 33(23):9752–68. doi: 10.1523/JNEUROSCI.0805-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fancy SP, Zhao C, Franklin RJ. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol Cell Neurosci. 2004;27(3):247–54. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Miller RH. Regulation of oligodendrocyte development in the vertebrate CNS. Prog Neurobiol. 2002;67(6):451–67. doi: 10.1016/s0301-0082(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 16.Almazan G, Honegger P, Matthieu JM. Triiodothyronine stimulation of oligodendroglial differentiation and myelination. A developmental study. Dev Neurosci. 1985;7(1):45–54. doi: 10.1159/000112275. [DOI] [PubMed] [Google Scholar]
- 17.D’Souza SD, Alinauskas KA, Antel JP. Ciliary neurotrophic factor selectively protects human oligodendrocytes from tumor necrosis factor-mediated injury. J Neurosci Res. 1996;43(3):289–98. doi: 10.1002/(SICI)1097-4547(19960201)43:3<289::AID-JNR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.McMorris FA, Dubois-Dalcq M. Insulin-like growth factor I promotes cell proliferation and oligodendroglial commitment in rat glial progenitor cells developing in vitro. J Neurosci Res. 1988;21(2–4):199–209. doi: 10.1002/jnr.490210212. [DOI] [PubMed] [Google Scholar]
- 19.Lebel C, et al. Diffusion tensor imaging of white matter tract evolution over the lifespan. Neuroimage. 2011;60(1):340–52. doi: 10.1016/j.neuroimage.2011.11.094. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal S, Yurlova L, Simons M. Central nervous system myelin: structure, synthesis and assembly. Trends Cell Biol. 2011;21(10):585–93. doi: 10.1016/j.tcb.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Franklin RJ, Ffrench-Constant C. Remyelination in the CNS: from biology to therapy. Nature reviews. Neuroscience. 2008;9(11):839–55. doi: 10.1038/nrn2480. [DOI] [PubMed] [Google Scholar]
- 22.Prineas JW, et al. Multiple sclerosis: remyelination of nascent lesions. Ann Neurol. 1993;33(2):137–51. doi: 10.1002/ana.410330203. [DOI] [PubMed] [Google Scholar]
- 23.Sim FJ, et al. Complementary patterns of gene expression by human oligodendrocyte progenitors and their environment predict determinants of progenitor maintenance and differentiation. Ann Neurol. 2006;59(5):763–79. doi: 10.1002/ana.20812. [DOI] [PubMed] [Google Scholar]
- 24.Horner PJ, Gage FH. Regenerating the damaged central nervous system. Nature. 2000;407(6807):963–70. doi: 10.1038/35039559. [DOI] [PubMed] [Google Scholar]
- 25.Menn B, et al. Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci. 2006;26(30):7907–18. doi: 10.1523/JNEUROSCI.1299-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nielsen HH, et al. Axonal degeneration stimulates the formation of NG2+ cells and oligodendrocytes in the mouse. Glia. 2006;54(2):105–15. doi: 10.1002/glia.20357. [DOI] [PubMed] [Google Scholar]
- 27.Blakemore WF, Irvine KA. Endogenous or exogenous oligodendrocytes for remyelination. J Neurol Sci. 2008;265(1–2):43–6. doi: 10.1016/j.jns.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 28.Kuhlmann T, et al. Differentiation block of oligodendroglial progenitor cells as a cause for remyelination failure in chronic multiple sclerosis. Brain. 2008;131(Pt 7):1749–58. doi: 10.1093/brain/awn096. [DOI] [PubMed] [Google Scholar]
- 29.Lucchinetti C, et al. A quantitative analysis of oligodendrocytes in multiple sclerosis lesions. A study of 113 cases. Brain. 1999;122(Pt 12):2279–95. doi: 10.1093/brain/122.12.2279. [DOI] [PubMed] [Google Scholar]
- 30.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci. 2002;3(9):705–14. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 31.Raine CS, et al. Demyelination in primate autoimmune encephalomyelitis and acute multiple sclerosis lesions: a case for antigen-specific antibody mediation. Ann Neurol. 1999;46(2):144–60. doi: 10.1002/1531-8249(199908)46:2<144::aid-ana3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 32.Franklin RJ, Kotter MR. The biology of CNS remyelination: the key to therapeutic advances. J Neurol. 2008;255(Suppl 1):19–25. doi: 10.1007/s00415-008-1004-6. [DOI] [PubMed] [Google Scholar]
- 33.Scolding N, et al. Oligodendrocyte progenitors are present in the normal adult human CNS and in the lesions of multiple sclerosis. Brain. 1998;121(Pt 12):2221–8. doi: 10.1093/brain/121.12.2221. [DOI] [PubMed] [Google Scholar]
- 34.Kipp M, et al. Multiple sclerosis: neuroprotective alliance of estrogen-progesterone and gender. Frontiers in neuroendocrinology. 2012;33(1):1–16. doi: 10.1016/j.yfrne.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Kipp M, van der Valk P, Amor S. Pathology of multiple sclerosis. CNS & neurological disorders drug targets. 2012;11(5):506–17. doi: 10.2174/187152712801661248. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, et al. Induction of oligodendrocyte differentiation by Olig2 and Sox10: evidence for reciprocal interactions and dosage-dependent mechanisms. Dev Biol. 2007;302(2):683–93. doi: 10.1016/j.ydbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Fancy SP, et al. Myelin regeneration: a recapitulation of development? Annu Rev Neurosci. 34:21–43. doi: 10.1146/annurev-neuro-061010-113629. [DOI] [PubMed] [Google Scholar]
- 38.Bibollet-Bahena O, Almazan G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J Neurochem. 2009;109(5):1440–51. doi: 10.1111/j.1471-4159.2009.06071.x. [DOI] [PubMed] [Google Scholar]
- 39.Narayanan SP, et al. Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination. J Neurosci. 2009;29(21):6860–70. doi: 10.1523/JNEUROSCI.0232-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tyler WA, et al. Activation of the mammalian target of rapamycin (mTOR) is essential for oligodendrocyte differentiation. J Neurosci. 2009;29(19):6367–78. doi: 10.1523/JNEUROSCI.0234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guardiola-Diaz HM, Ishii A, Bansal R. Erk1/2 MAPK and mTOR signaling sequentially regulates progression through distinct stages of oligodendrocyte differentiation. Glia. 2012;60(3):476–86. doi: 10.1002/glia.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherman DL, et al. Arrest of myelination and reduced axon growth when Schwann cells lack mTOR. J Neurosci. 2012;32(5):1817–25. doi: 10.1523/JNEUROSCI.4814-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biotechnol. 2005;31(2):151–74. doi: 10.1385/MB:31:2:151. [DOI] [PubMed] [Google Scholar]
- 44.Ishii A, et al. Role of ERK1/2 MAPK signaling in the maintenance of myelin and axonal integrity in the adult CNS. J Neurosci. 2014;34(48):16031–45. doi: 10.1523/JNEUROSCI.3360-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takebayashi H, Ikenaka K. Oligodendrocyte generation during mouse development. Glia. 2015;63(8):1350–6. doi: 10.1002/glia.22863. [DOI] [PubMed] [Google Scholar]
- 46.Lopez Juarez A, He D, Richard Lu Q. Oligodendrocyte progenitor programming and reprogramming: Toward myelin regeneration. Brain Res. 2015 doi: 10.1016/j.brainres.2015.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maeda Y, et al. Platelet-derived growth factor-alpha receptor-positive oligodendroglia are frequent in multiple sclerosis lesions. Ann Neurol. 2001;49(6):776–85. doi: 10.1002/ana.1015. [DOI] [PubMed] [Google Scholar]
- 48.Hinks GL, Franklin RJ. Distinctive patterns of PDGF-A, FGF-2, IGF-I, and TGF-beta1 gene expression during remyelination of experimentally-induced spinal cord demyelination. Mol Cell Neurosci. 1999;14(2):153–68. doi: 10.1006/mcne.1999.0771. [DOI] [PubMed] [Google Scholar]
- 49.Blaschuk KL, Frost EE, ffrench-Constant C. The regulation of proliferation and differentiation in oligodendrocyte progenitor cells by alphaV integrins. Development. 2000;127(9):1961–9. doi: 10.1242/dev.127.9.1961. [DOI] [PubMed] [Google Scholar]
- 50.Bonora M, et al. Tumor necrosis factor-alpha impairs oligodendroglial differentiation through a mitochondria-dependent process. Cell Death Differ. 2014;21(8):1198–208. doi: 10.1038/cdd.2014.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mason JL, et al. Interleukin-1beta promotes repair of the CNS. J Neurosci. 2001;21(18):7046–52. doi: 10.1523/JNEUROSCI.21-18-07046.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel JR, et al. CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A. 2010;107(24):11062–7. doi: 10.1073/pnas.1006301107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van der Pal RH, et al. Effects of insulin and insulin-like growth factor (IGF-I) on oligodendrocyte-enriched glial cultures. J Neurosci Res. 1988;19(4):483–90. doi: 10.1002/jnr.490190412. [DOI] [PubMed] [Google Scholar]
- 54.Xiu-li W, et al. ERB-041, a selective ER beta agonist, inhibits iNOS production in LPS-activated peritoneal macrophages of endometriosis via suppression of NF-kappaB activation. Mol Immunol. 2009;46(11–12):2413–8. doi: 10.1016/j.molimm.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 55.Fressinaud C, Vallat JM, Labourdette G. Basic fibroblast growth factor down-regulates myelin basic protein gene expression and alters myelin compaction of mature oligodendrocytes in vitro. J Neurosci Res. 1995;40(3):285–93. doi: 10.1002/jnr.490400302. [DOI] [PubMed] [Google Scholar]
- 56.Dubois-Dalcq M, Murray K. Why are growth factors important in oligodendrocyte physiology? Pathol Biol (Paris) 2000;48(1):80–6. [PubMed] [Google Scholar]
- 57.Carson MJ, et al. Insulin-like growth factor I increases brain growth and central nervous system myelination in transgenic mice. Neuron. 1993;10(4):729–40. doi: 10.1016/0896-6273(93)90173-o. [DOI] [PubMed] [Google Scholar]
- 58.Zeger M, et al. Insulin-like growth factor type 1 receptor signaling in the cells of oligodendrocyte lineage is required for normal in vivo oligodendrocyte development and myelination. Glia. 2007;55(4):400–11. doi: 10.1002/glia.20469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Charles P, et al. Re-expression of PSA-NCAM by demyelinated axons: an inhibitor of remyelination in multiple sclerosis? Brain. 2002;125(Pt 9):1972–9. doi: 10.1093/brain/awf216. [DOI] [PubMed] [Google Scholar]
- 60.Hu QD, et al. F3/contactin acts as a functional ligand for Notch during oligodendrocyte maturation. Cell. 2003;115(2):163–75. doi: 10.1016/s0092-8674(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 61.Shimizu T, et al. Wnt signaling controls the timing of oligodendrocyte development in the spinal cord. Dev Biol. 2005;282(2):397–410. doi: 10.1016/j.ydbio.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Mosyak L, et al. The structure of the Lingo-1 ectodomain, a module implicated in central nervous system repair inhibition. J Biol Chem. 2006;281(47):36378–90. doi: 10.1074/jbc.M607314200. [DOI] [PubMed] [Google Scholar]
- 63.Clarner T, et al. Myelin debris regulates inflammatory responses in an experimental demyelination animal model and multiple sclerosis lesions. Glia. 2012;60(10):1468–80. doi: 10.1002/glia.22367. [DOI] [PubMed] [Google Scholar]
- 64.Preston M, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73(2):266–80. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lopez-Diego RS, Weiner HL. Novel therapeutic strategies for multiple sclerosis--a multifaceted adversary. Nat Rev Drug Discov. 2008;7(11):909–25. doi: 10.1038/nrd2358. [DOI] [PubMed] [Google Scholar]
- 66.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9(6):393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y, et al. Inhibition of LINGO-1 promotes functional recovery after experimental spinal cord demyelination. Exp Neurol. 2015;266:68–73. doi: 10.1016/j.expneurol.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Bourikas D, Mir A, Walmsley AR. LINGO-1-mediated inhibition of oligodendrocyte differentiation does not require the leucine-rich repeats and is reversed by p75(NTR) antagonists. Mol Cell Neurosci. 2010;45(4):363–9. doi: 10.1016/j.mcn.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 69.Bhatt A, Fan LW, Pang Y. Strategies for myelin regeneration: lessons learned from development. Neural Regen Res. 2014;9(14):1347–50. doi: 10.4103/1673-5374.137586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Najm FJ, et al. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522(7555):216–20. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arevalo MA, Azcoitia I, Garcia-Segura LM. The neuroprotective actions of oestradiol and oestrogen receptors. Nat Rev Neurosci. 2015;16(1):17–29. doi: 10.1038/nrn3856. [DOI] [PubMed] [Google Scholar]
- 72.Amantea D, et al. From clinical evidence to molecular mechanisms underlying neuroprotection afforded by estrogens. Pharmacol Res. 2005;52(2):119–32. doi: 10.1016/j.phrs.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Johann S, Beyer C. Neuroprotection by gonadal steroid hormones in acute brain damage requires cooperation with astroglia and microglia. J Steroid Biochem Mol Biol. 2013;137:71–81. doi: 10.1016/j.jsbmb.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, et al. Estrogen actions in the brain. Sci STKE. 2002;2002(138):pe29. doi: 10.1126/stke.2002.138.pe29. [DOI] [PubMed] [Google Scholar]
- 75.Tedesco R, et al. The estrogen receptor: a structure-based approach to the design of new specific hormone-receptor combinations. Chem Biol. 2001;8(3):277–87. doi: 10.1016/s1074-5521(01)00006-0. [DOI] [PubMed] [Google Scholar]
- 76.Cunningham M, Gilkeson G. Estrogen receptors in immunity and autoimmunity. Clin Rev Allergy Immunol. 40(1):66–73. doi: 10.1007/s12016-010-8203-5. [DOI] [PubMed] [Google Scholar]
- 77.Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol Med Microbiol. 2003;38(1):13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- 78.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–84. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 79.Abramsky O. Pregnancy and multiple sclerosis. Ann Neurol. 1994;36(Suppl):S38–41. doi: 10.1002/ana.410360712. [DOI] [PubMed] [Google Scholar]
- 80.Confavreux C, et al. Rate of pregnancy-related relapse in multiple sclerosis. Pregnancy in Multiple Sclerosis Group. N Engl J Med. 1998;339(5):285–91. doi: 10.1056/NEJM199807303390501. [DOI] [PubMed] [Google Scholar]
- 81.Da Silva JA, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clin Rheumatol. 1992;11(2):189–94. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 82.Hellwig K. Pregnancy in multiple sclerosis. Eur Neurol. 2014;72(Suppl 1):39–42. doi: 10.1159/000367640. [DOI] [PubMed] [Google Scholar]
- 83.Miller DH, et al. Pregnancy, sex and hormonal factors in multiple sclerosis. Mult Scler. 2014;20(5):527–36. doi: 10.1177/1352458513519840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S, et al. Estriol ameliorates autoimmune demyelinating disease: implications for multiple sclerosis. Neurology. 1999;52(6):1230–8. doi: 10.1212/wnl.52.6.1230. [DOI] [PubMed] [Google Scholar]
- 85.Bebo BF, Jr, et al. Low-dose estrogen therapy ameliorates experimental autoimmune encephalomyelitis in two different inbred mouse strains. J Immunol. 2001;166(3):2080–9. doi: 10.4049/jimmunol.166.3.2080. [DOI] [PubMed] [Google Scholar]
- 86.Jansson L, Olsson T, Holmdahl R. Estrogen induces a potent suppression of experimental autoimmune encephalomyelitis and collagen-induced arthritis in mice. J Neuroimmunol. 1994;53(2):203–7. doi: 10.1016/0165-5728(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 87.Sicotte NL, et al. Treatment of multiple sclerosis with the pregnancy hormone estriol. Ann Neurol. 2002;52(4):421–8. doi: 10.1002/ana.10301. [DOI] [PubMed] [Google Scholar]
- 88.Voskuhl RR, et al. Estriol combined with glatiramer acetate for women with relapsing-remitting multiple sclerosis: a randomised, placebo-controlled, phase 2 trial. Lancet Neurol. 2016;15(1):35–46. doi: 10.1016/S1474-4422(15)00322-1. [DOI] [PubMed] [Google Scholar]
- 89.Prokai L, et al. The prodrug DHED selectively delivers 17beta-estradiol to the brain for treating estrogen-responsive disorders. Sci Transl Med. 2015;7(297):297ra113. doi: 10.1126/scitranslmed.aab1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burns KA, Korach KS. Estrogen receptors and human disease: an update. Arch Toxicol. 2012;86(10):1491–504. doi: 10.1007/s00204-012-0868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Z, et al. Comparison of in vivo and in vitro subcellular localization of estrogen receptors alpha and beta in oligodendrocytes. J Neurochem. 2004;89(3):674–84. doi: 10.1111/j.1471-4159.2004.02388.x. [DOI] [PubMed] [Google Scholar]
- 92.Takao T, et al. 17beta-estradiol protects oligodendrocytes from cytotoxicity induced cell death. J Neurochem. 2004;89(3):660–73. doi: 10.1111/j.1471-4159.2004.02370.x. [DOI] [PubMed] [Google Scholar]
- 93.Khalaj AJ, et al. Estrogen receptor (ER) beta expression in oligodendrocytes is required for attenuation of clinical disease by an ERbeta ligand. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(47):19125–30. doi: 10.1073/pnas.1311763110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kumar S, et al. Estrogen receptor beta ligand therapy activates PI3K/Akt/mTOR signaling in oligodendrocytes and promotes remyelination in a mouse model of multiple sclerosis. Neurobiology of disease. 2013;56:131–44. doi: 10.1016/j.nbd.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hirahara Y, et al. The localization and non-genomic function of the membrane-associated estrogen receptor in oligodendrocytes. Glia. 2009;57(2):153–65. doi: 10.1002/glia.20742. [DOI] [PubMed] [Google Scholar]
- 96.Meltser I, et al. Estrogen receptor beta protects against acoustic trauma in mice. J Clin Invest. 2008;118(4):1563–70. doi: 10.1172/JCI32796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Van’t Veer A, et al. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J Neurosci Res. 2009;87(1):69–78. doi: 10.1002/jnr.21841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vondran MW, et al. BDNF+/− mice exhibit deficits in oligodendrocyte lineage cells of the basal forebrain. Glia. 2010;58(7):848–56. doi: 10.1002/glia.20969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wood TL, et al. mTOR: a link from the extracellular milieu to transcriptional regulation of oligodendrocyte development. ASN Neuro. 2013;5(1):e00108. doi: 10.1042/AN20120092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tiwari-Woodruff S, Voskuhl RR. Neuroprotective and anti-inflammatory effects of estrogen receptor ligand treatment in mice. Journal of the neurological sciences. 2009;286(1–2):81–5. doi: 10.1016/j.jns.2009.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morales LB, et al. Treatment with an estrogen receptor alpha ligand is neuroprotective in experimental autoimmune encephalomyelitis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26(25):6823–33. doi: 10.1523/JNEUROSCI.0453-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tiwari-Woodruff S, et al. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14813–8. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moore SM, et al. Multiple functional therapeutic effects of the estrogen receptor β agonist indazole-Cl in a mouse model of multiple sclerosis. Proc Natl Acad Sci U S A. 2014;111(50):18061–6. doi: 10.1073/pnas.1411294111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mohler ML, et al. Estrogen receptor beta selective nonsteroidal estrogens: seeking clinical indications. Expert Opin Ther Pat. 2010;20(4):507–34. doi: 10.1517/13543771003657164. [DOI] [PubMed] [Google Scholar]
- 105.Stovall DW, Pinkerton JV. MF-101, an estrogen receptor beta agonist for the treatment of vasomotor symptoms in peri- and postmenopausal women. Curr Opin Investig Drugs. 2009;10(4):365–71. [PubMed] [Google Scholar]
- 106.Paruthiyil S, et al. Drug and cell type-specific regulation of genes with different classes of estrogen receptor beta-selective agonists. PLoS One. 2009;4(7):e6271. doi: 10.1371/journal.pone.0006271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Minutolo F, et al. Estrogen receptor beta ligands: recent advances and biomedical applications. Med Res Rev. 2011;31(3):364–442. doi: 10.1002/med.20186. [DOI] [PubMed] [Google Scholar]
- 108.Carroll VM, et al. Diarylpropionitrile (DPN) Enantiomers: Synthesis and Evaluation of Estrogen Receptor beta-Selective Ligands. J Med Chem. 2011;55(1):528–37. doi: 10.1021/jm201436k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harris HA, et al. Evaluation of an estrogen receptor-beta agonist in animal models of human disease. Endocrinology. 2003;144(10):4241–9. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 110.Liu F, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nature neuroscience. 2008;11(3):334–43. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 111.Cvoro A, et al. Selective estrogen receptor-beta agonists repress transcription of proinflammatory genes. J Immunol. 2008;180(1):630–6. doi: 10.4049/jimmunol.180.1.630. [DOI] [PubMed] [Google Scholar]
- 112.Harris HA. Preclinical characterization of selective estrogen receptor beta agonists: new insights into their therapeutic potential. Ernst Schering Found Symp Proc. 2006;(1):149–61. doi: 10.1007/2789_2006_021. [DOI] [PubMed] [Google Scholar]
- 113.Elloso MM, et al. Suppression of experimental autoimmune encephalomyelitis using estrogen receptor-selective ligands. J Endocrinol. 2005;185(2):243–52. doi: 10.1677/joe.1.06063. [DOI] [PubMed] [Google Scholar]
- 114.Roman-Blas JA, et al. Efficacy and safety of a selective estrogen receptor-beta agonist, ERB-041, in patients with rheumatoid arthritis. Arthritis Care Res (Hoboken) 2010 doi: 10.1002/acr.20275. [DOI] [PubMed] [Google Scholar]
- 115.Srivastava DP, et al. Estrogen receptor β activity modulates synaptic signaling and structure. J Neurosci. 2010;30(40):13454–60. doi: 10.1523/JNEUROSCI.3264-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Saijo K, et al. An ADIOL-ERbeta-CtBP transrepression pathway negatively regulates microglia-mediated inflammation. Cell. 2011;145(4):584–95. doi: 10.1016/j.cell.2011.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Meyers MJ, et al. Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues. J Med Chem. 2001;44(24):4230–51. doi: 10.1021/jm010254a. [DOI] [PubMed] [Google Scholar]
- 118.Weiser MJ, Wu TJ, Handa RJ. Estrogen receptor-beta agonist diarylpropionitrile: biological activities of R- and S-enantiomers on behavior and hormonal response to stress. Endocrinology. 2009;150(4):1817–25. doi: 10.1210/en.2008-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Crawford DK, et al. Oestrogen receptor beta ligand: a novel treatment to enhance endogenous functional remyelination. Brain: a journal of neurology. 2010;133(10):2999–3016. doi: 10.1093/brain/awq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tiwari-Woodruff SK, et al. Electrostatic interactions between transmembrane segments mediate folding of Shaker K+ channel subunits. Biophysical journal. 1997;72(4):1489–500. doi: 10.1016/S0006-3495(97)78797-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Spence RD, et al. Estrogen Mediates Neuroprotection and Anti-Inflammatory Effects during EAE through ERalpha Signaling on Astrocytes But Not through ERbeta Signaling on Astrocytes or Neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33(26):10924–33. doi: 10.1523/JNEUROSCI.0886-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.De Angelis M, et al. Indazole estrogens: highly selective ligands for the estrogen receptor beta. J Med Chem. 2005;48(4):1132–44. doi: 10.1021/jm049223g. [DOI] [PubMed] [Google Scholar]
- 123.Zhao L, Woody SK, Chhibber A. Estrogen receptor β in Alzheimer’s disease: From mechanisms to therapeutics. Ageing Res Rev. 2015;24(Pt B):178–90. doi: 10.1016/j.arr.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bonetti A, et al. A follow-up study of chromosome 19q13 in multiple sclerosis susceptibility. J Neuroimmunol. 2009;208(1–2):119–24. doi: 10.1016/j.jneuroim.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nicoletti F, et al. 5-androstenediol ameliorates pleurisy, septic shock, and experimental autoimmune encephalomyelitis in mice. Autoimmune Dis. 2010;2010:757432. doi: 10.4061/2010/757432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wu WF, et al. Targeting estrogen receptor beta in microglia and T cells to treat experimental autoimmune encephalomyelitis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(9):3543–8. doi: 10.1073/pnas.1300313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Harris HA, et al. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20(4):936–41. doi: 10.1093/humrep/deh711. [DOI] [PubMed] [Google Scholar]
- 128.Christaki E, et al. Estrogen receptor beta agonism increases survival in experimentally induced sepsis and ameliorates the genomic sepsis signature: a pharmacogenomic study. J Infect Dis. 2010;201(8):1250–7. doi: 10.1086/651276. [DOI] [PubMed] [Google Scholar]