Abstract
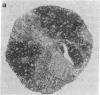
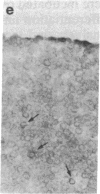
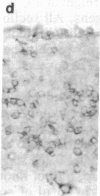
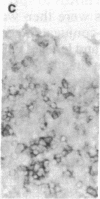
Using differential radiation sensitivity of components of mouse embryonal thymus, an in vitro method for studying T-cell differentiation from hemopoietic stem cells (HSCs) in the G0 phase was established. Intrathymic T-cell precursors present in embryonal thymus were found to be quite radioresistant (up to 20 Gy), and consequently 25-Gy-irradiated embryonal thymic lobes were used. Thymic lobes (25-Gy irradiated) taken from mouse fetuses (gestation day 15) were placed in Millipore-HA culture plates supported on squares of gelatin foam sponge in 24-well culture plates in which neonatal thymus stromal cells were cultured. HSCs (10(5) cells per well) in the G0 phase were added to these thymic lobes and cocultured at 37 degrees C in a 5% CO2/95% air incubator. Half the culture medium was changed every week. After 3 weeks, a large number of colonies had formed. Immunohistochemical studies and fluorescence-activated cell sorter analyses revealed that the colonizing cells regularly develop and exhibit surface markers characteristic of T cells (Thy-1, IL-2R, L3T4, Lyt-2, etc.). In situ hybridization analyses revealed that mRNA expression for T-cell receptor (TCR) beta chains occurred within colonizing cells. Using a monoclonal antibody (F23.1), expression of TCR beta-chain variable domain (V beta 8) on the surface of these developing T cells was demonstrated. These cells responded to interleukin 2 and/or anti-CD3 monoclonal antibody, indicating functional T cells. This method will be useful in studying T-cell differentiation pathways from pluripotent HSCs and in clarifying the mechanisms involved in negative and positive selection of T cells within the thymus.
Full text
PDF
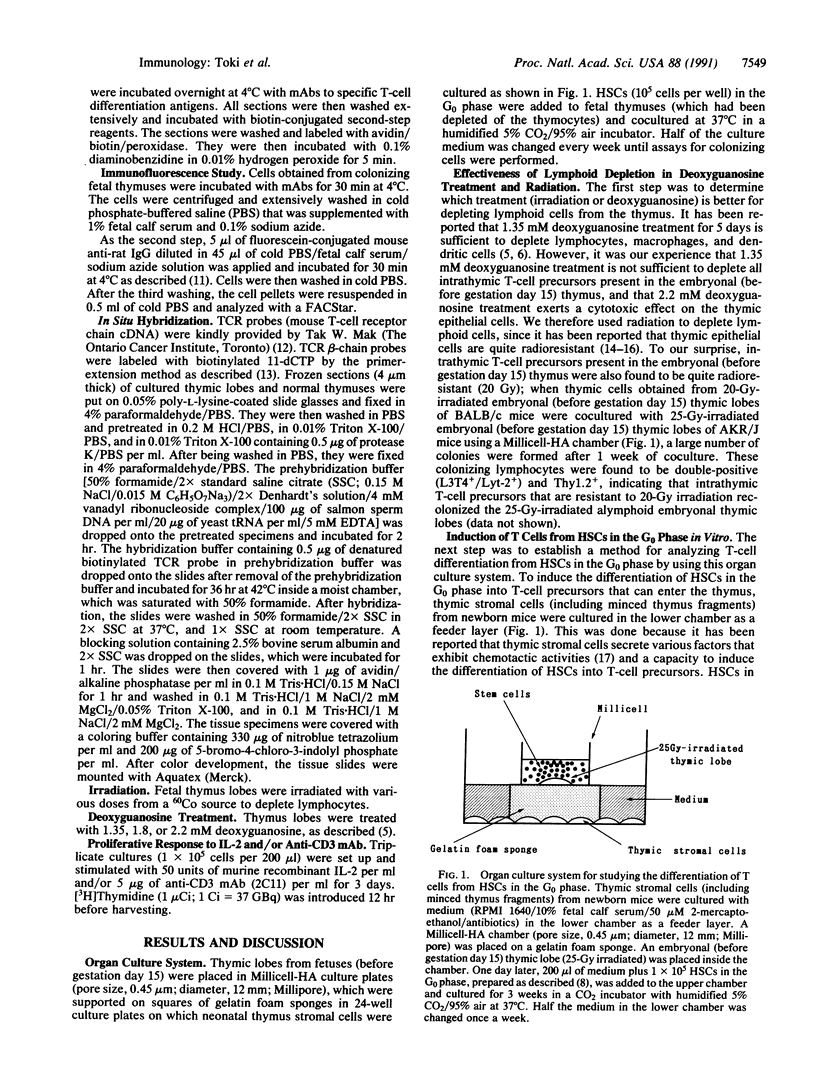
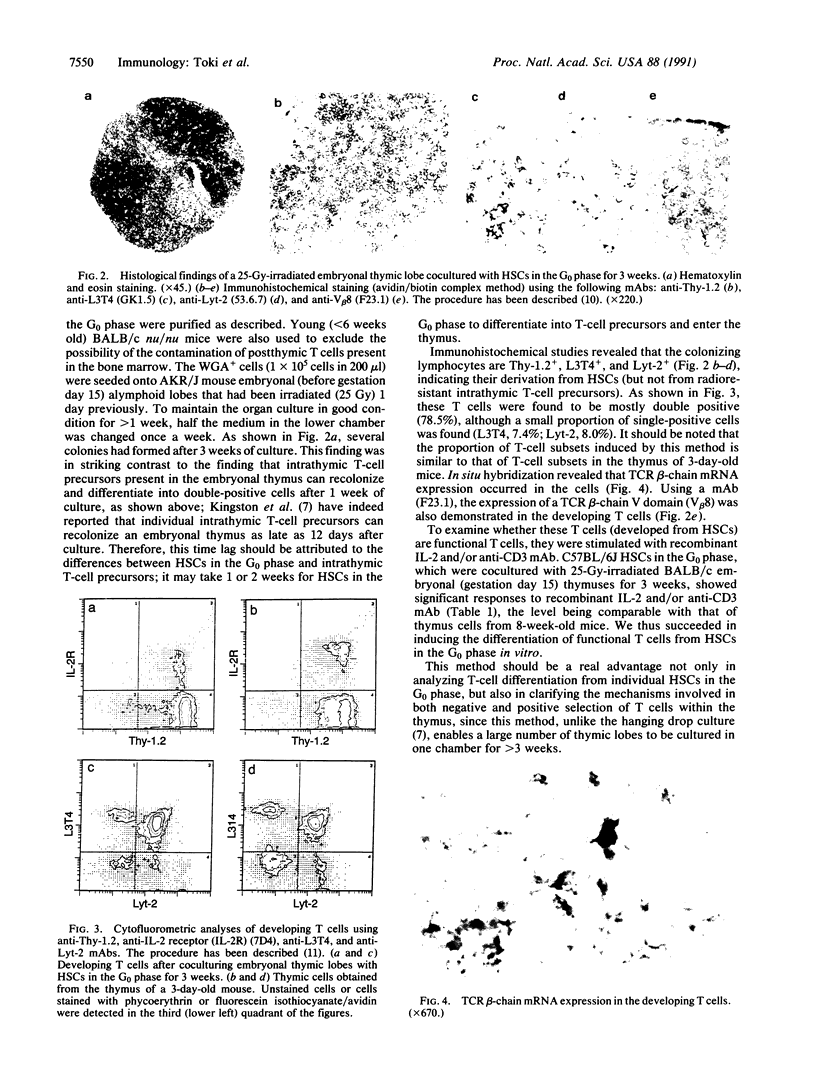

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARCHER O. K., PIERCE J. C., PAPERMASTER B. W., GOOD R. A. Reduced antibody response in thymectomized rabbits. Nature. 1962 Jul 14;195:191–193. doi: 10.1038/195191a0. [DOI] [PubMed] [Google Scholar]
- Caccia N., Kronenberg M., Saxe D., Haars R., Bruns G. A., Goverman J., Malissen M., Willard H., Yoshikai Y., Simon M. The T cell receptor beta chain genes are located on chromosome 6 in mice and chromosome 7 in humans. Cell. 1984 Jul;37(3):1091–1099. doi: 10.1016/0092-8674(84)90443-4. [DOI] [PubMed] [Google Scholar]
- Champion S., Imhof B. A., Savagner P., Thiery J. P. The embryonic thymus produces chemotactic peptides involved in the homing of hemopoietic precursors. Cell. 1986 Mar 14;44(5):781–790. doi: 10.1016/0092-8674(86)90844-5. [DOI] [PubMed] [Google Scholar]
- Davis W. E., Jr, Cole L. J. Retarded immunological recovery in sublethally x-irradiated mice by additional thymic exposure. Reversal with injected marrow cells. Proc Soc Exp Biol Med. 1969 Apr;130(4):1336–1344. doi: 10.3181/00379727-130-33787. [DOI] [PubMed] [Google Scholar]
- GOOD R. A., DALMASSO A. P., MARTINEZ C., ARCHER O. K., PIERCE J. C., PAPERMASTER B. W. The role of the thymus in development of immunologic capacity in rabbits and mice. J Exp Med. 1962 Nov 1;116:773–796. doi: 10.1084/jem.116.5.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa K., Sado T. Radiation effects on regeneration and T-cell-inducing function of the thymus. Cell Immunol. 1984 Apr 1;84(2):372–379. doi: 10.1016/0008-8749(84)90109-6. [DOI] [PubMed] [Google Scholar]
- Ikehara S., Yasumizu R., Inaba M., Izui S., Hayakawa K., Sekita K., Toki J., Sugiura K., Iwai H., Nakamura T. Long-term observations of autoimmune-prone mice treated for autoimmune disease by allogeneic bone marrow transplantation. Proc Natl Acad Sci U S A. 1989 May;86(9):3306–3310. doi: 10.1073/pnas.86.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Franchi L. L., Kingston R., Owen J. J. Effect of deoxyguanosine on lymphopoiesis in the developing thymus rudiment in vitro: application in the production of chimeric thymus rudiments. Eur J Immunol. 1982 Jul;12(7):583–587. doi: 10.1002/eji.1830120710. [DOI] [PubMed] [Google Scholar]
- Kingston R., Jenkinson E. J., Owen J. J. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. 1985 Oct 31-Nov 6Nature. 317(6040):811–813. doi: 10.1038/317811a0. [DOI] [PubMed] [Google Scholar]
- Low T. L., Goldstein A. L. Thymic hormones: an overview. Methods Enzymol. 1985;116:213–219. doi: 10.1016/s0076-6879(85)16015-5. [DOI] [PubMed] [Google Scholar]
- MILLER J. F. Immunological function of the thymus. Lancet. 1961 Sep 30;2(7205):748–749. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- Miyama-Inaba M., Kuma S., Inaba K., Ogata H., Iwai H., Yasumizu R., Muramatsu S., Steinman R. M., Ikehara S. Unusual phenotype of B cells in the thymus of normal mice. J Exp Med. 1988 Aug 1;168(2):811–816. doi: 10.1084/jem.168.2.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyama-Inaba M., Ogata H., Toki J., Kuma S., Sugiura K., Yasumizu R., Ikehara S. Isolation of murine pluripotent hemopoietic stem cells in the Go phase. Biochem Biophys Res Commun. 1987 Sep 15;147(2):687–694. doi: 10.1016/0006-291x(87)90985-5. [DOI] [PubMed] [Google Scholar]
- Ready A. R., Jenkinson E. J., Kingston R., Owen J. J. Successful transplantation across major histocompatibility barrier of deoxyguanosine-treated embryonic thymus expressing class II antigens. Nature. 1984 Jul 19;310(5974):231–233. doi: 10.1038/310231a0. [DOI] [PubMed] [Google Scholar]
- Visser J. W., Van Bekkum D. W. Purification of pluripotent hemopoietic stem cells: past and present. Exp Hematol. 1990 Mar;18(3):248–256. [PubMed] [Google Scholar]