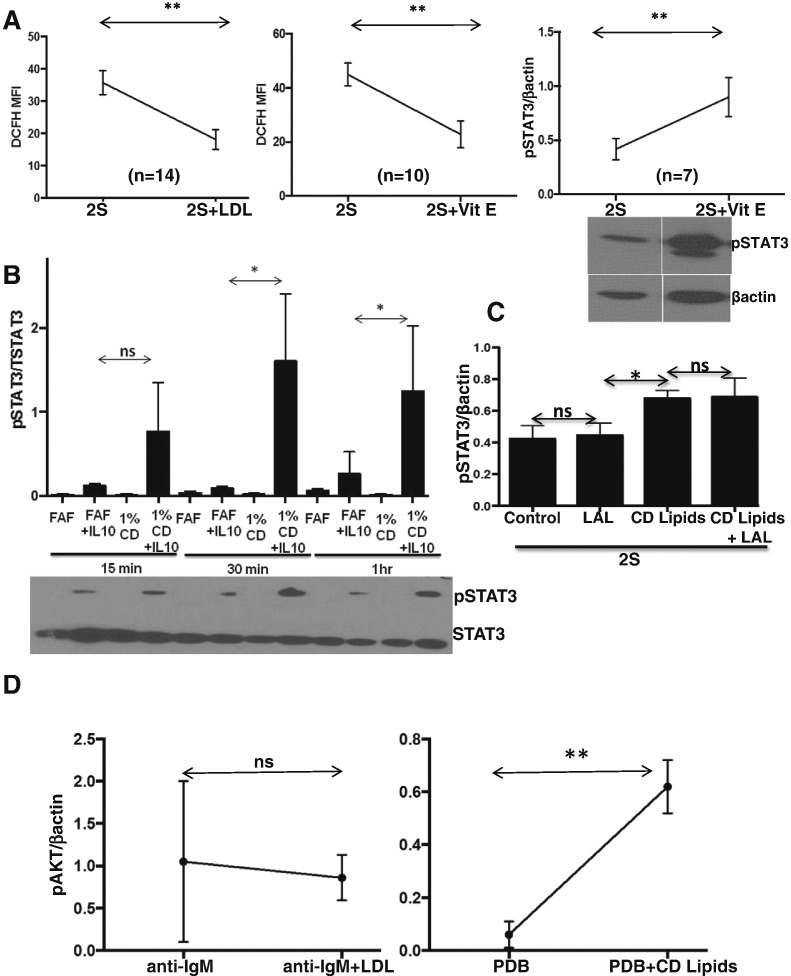
Fig. 5.
Effect of LDL components on oxidative stress and signaling in CLL cells. A. CLL cells were activated with IL2 and resiquimod (2S) and with or without LDL (0.5 mM) or Vitamin E (5 μM). Mean fluorescence intensities (MFIs) of DCFH staining were determined by flow cytometry after 12 h (left and middle panels) and densitometric values of p-STAT3 expression relative to β-actin were determined after 18 h (right panel). Shown are the average results and standard errors from the number of patient samples indicated in each graph. An example of an immunoblot is shown in the insert. B. Purified CLL cells (1 × 106 cells/ml) were cultured over-night in fatty acid free media (FAF) or 1% CD Lipid extract, consisting of a mixture of free cholesterol, fatty acids, and Vitamin E. The cells were then treated with IL10 (10 ng/ml) but not otherwise activated with IL2 and resiquimod. Levels of pSTAT3 were measured by immunoblotting after 15 min, 30 min, and 1 h using STAT3 as the loading control. Averages and standard deviations of relative pSTAT3 densitometry values for 6 different patient samples are plotted with a representative immunoblot shown below the graph. C. Relative pSTAT3 values were measured in CLL cells from 6 different patients that had been activated with IL2 and resiquimod for 18 h in fatty acid free media (FAF) or 1% CD Lipid extract with or without Lalistat (LAL) (1 μM). Averages and standard deviations are shown. D. CLL cells were cultured with or without LDL (0.5 mM) for 18 h and stimulated with anti-IgM antibodies (10 ng/ml) (n = 3; left panel) or phorbol dibutyrate (5 ng/ml) (n = 3; right panel). Phospho-AKT (pAKT) levels were determined after 30 min by immunoblotting with β-actin as a loading control. *p < 0.05; **p < 0.01; ns, non significant.