Abstract
To investigate the effects of micronutrients on blood pressure (BP) in patients with type 2 diabetes through a systematic review and meta-analysis, randomized clinical trials (RCTs) of the effects of individual micronutrients on BP in patients with type 2 diabetes were searched in the Medline, Embase, Cochrane, and Clinical Trials.gov databases through April 2016. From the 28,164 studies, 11 RCTs (13 interventions, 723 patients, 54% males) with 3 to 52 weeks of follow-up were classified according to the type of micronutrient intervention: sodium (n = 1), vitamin C (n = 2), vitamin D (n = 7), and magnesium (n = 1). The available data enabled us to perform meta-analyses of vitamins C and D. Vitamin C reduced diastolic BP [WMD −2.88 mmHg (95%CI −5.31, −0.46; P = 0.020)] but not systolic BP [WMD −3.93 mmHg (95%CI −14.78, 6.92; P = 0.478)]. Vitamin D caused a reduction of 4.56 mmHg (WMD; 95%CI −7.65, −1.47; P = 0.004) for systolic BP and 2.44 mm Hg (WMD; 95%CI −3.49, −1.39; P < 0.001) for diastolic BP. In conclusion, vitamin D and possibly vitamin C have beneficial effects on BP in patients with type 2 diabetes. These interventions might represent a novel approach to the treatment of hypertension in these patients.
Hypertension is a major risk factor for chronic complications of diabetes1, and approximately 71% of patients with type 2 diabetes in the United States have hypertension2. Indeed, most hypertensive type 2 diabetic patients do not achieve optimal blood pressure (BP) levels3. Dietary interventions can reduce BP, prevent or delay the development of hypertension, enhance antihypertensive drug efficacy, and decrease cardiovascular risk4,5, and this evidence highlights the potential therapeutic role of dietary interventions in the management of hypertension.
Dietary recommendations for patients with hypertension include the reduction of sodium intake, moderation of alcohol consumption, and the Dietary Approaches to Stop Hypertension (DASH) eating plan6,7,8 recommended by the American Diabetes Association (ADA)9. However, dietary advice for hypertension in diabetes4,6,9,10,11 has been mostly based on studies conducted in non-diabetic subjects5,7,8. In patients with type 2 diabetes, the beneficial association of the DASH diet with BP was previously demonstrated in both a cross-sectional study12 and clinical trials13,14.
The relationship between individual micronutrients and BP in patients with type 2 diabetes mellitus is still uncertain. Data from individual studies15,16,17,18,19,20,21,22,23,24,25 may not be sufficient to clearly demonstrate the effects of micronutrients on BP. Therefore, the aim of the current study was to evaluate the impact of individual micronutrients on BP in patients with type 2 diabetes through a systematic review and meta-analysis of randomized clinical trials (RCTs).
Methods
This systematic review was carried out using a protocol designed according to the Cochrane Handbook recommendations26 and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement27.
Data sources and search strategy
We searched the Medline, Embase, Cochrane and ClinicalTrials.gov electronic databases to identify RCTs that reported the effects of micronutrients on BP in patients with type 2 diabetes, with or without hypertension, through April 2016. The initial search strategy is available in the Supplementary Information. The following medical subject headings (MeSH) terms were used: “Blood Pressure” OR “Hypertension” AND “Diabetes Mellitus” AND Micronutrients” OR “Sodium” OR “Magnesium” OR “Ascorbic Acid” OR “Vitamin D”. We also used MeSH terms for macronutrients (carbohydrates, proteins, lipids) to perform a more comprehensive search in addition to the terms related to micronutrients.
Study selection
Studies were considered eligible for inclusion if they fulfilled all of the following inclusion criteria: presented original data from RCTs assessing the effects of dietary or oral supplementation of a micronutrient on BP values in patients with type 2 diabetes, examined the effect of a micronutrient on BP after a minimum of two weeks of intervention, and reported means (or differences between means) and standard deviations (SD) of BP at baseline and after the intervention. We excluded studies involving children or pregnant women, as well as studies that evaluated more than one micronutrient intervention at the same time or the simultaneous effects of exercise, genetic polymorphisms, and caloric restriction. We also excluded studies that evaluated a specific dietary pattern instead of an individual micronutrient. Crossover trials were excluded if BP was not evaluated before and after each dietary intervention or if there was no description of a washout period between the studied diets.
Hypertension was defined as a systolic BP ≥ 140 mmHg or a diastolic BP ≥ 90 mmHg and/or the use of antihypertensive medication4. Studies involving patients who were using antihypertensive medication were not excluded unless the antihypertensive medication was used as an intervention in addition to the supplement.
Data extraction and quality assessment
Two reviewers (T.P.P. and C.K.K.) independently analysed the titles and abstracts of every paper retrieved by the literature search to identify potentially eligible studies. All articles that did not meet the selection criteria were excluded, and the full text of the remaining papers was obtained for further examination. The reference lists of the retrieved articles were also manually searched (T.P.P. and C.K.K.) for all included studies. The data were extracted independently by the same two investigators with excellent agreement between them (k = 97%). Any disagreements were resolved by a third author (M.J.A. or L.V.V.).
The extracted data (in addition to assessment of office BP changes as a mean (SD), primary or secondary outcome) included the following: type of micronutrient intervention (dietary or supplemental), author’s name, year of publication, trial design, duration of intervention, number of participants, patient characteristics (gender, age, percentage of patients with hypertension, diabetes duration, BMI), and medication use. Information regarding diet characteristics (total energy and macronutrient intake) and evaluation of dietary compliance were extracted from the intervention and control diet descriptions when available.
A quality assessment of our systematic review was performed in order to limit any bias in conducting the study, gain insight into potential comparisons, and guide the interpretation of the findings26. To evaluate the quality assessment, two reviewers (T.P. and M.J.A) independently assessed the quality of the methodology of each of the studies included in our systematic review. We used a score based on the Cochrane Collaboration Handbook to assess the individual risk of bias of every included study26,28. The biases were classified into six categories: selection, performance, detection, attrition, reporting, and other bias26,27,28. We included the assessment of dietary compliance in the “other bias” category. The risk of bias for each individual item was classified as high, low, or unclear. Regarding dietary compliance, the risk of bias was classified as “low” if the study described the method of dietary compliance evaluation.
The quality of the body of evidence in our systematic review was assessed by taking into account the GRADE26,29 recommendations. The GRADE results provide an optimal primary approach to making decisions regarding imprecision29 and allowed us to accurately evaluate the relevance of our systematic review. The GRADE approach includes factors that may decrease (e.g., poor methodological quality, indirectness of evidence, high heterogeneity, imprecision of effect estimates, high risk of publication bias) or increase (e.g., large effect size, absence of spurious effects due to confounding factors, description of the dose-response gradient) the quality of the evidence. Each evaluated factor was rated as high, moderate, low, or very low quality26,28,29. Using this approach, a serious risk of bias was considered if an individual study had more than three unclear or high risk of bias. Imprecision was defined as a meta-analysis confidence interval >0.5. The GRADE quality was evaluated using GRADEpro version 3.6.1 (2004–2001), and all topics of the performed meta-analyses were analysed by the PRISMA checklist27.
Data synthesis and analysis
The descriptive data from the systematic review are presented as mean and/or range whenever possible. Changes in BP for each analysed micronutrient intervention are reported as absolute differences between the arithmetic means at end-of-study and baseline, and weighted mean differences (WMD) were used in the analyses. Changes in the standard deviation (SD) values of BP were directly extracted from the manuscripts or calculated. When SD data were not included, they were calculated, and a correlation of 0.8 between the baseline and final measurements within each group was assumed according to the formula of Follman et al.30, as proposed by the Cochrane Handbook30. We assumed equal variance among the trials and between the intervention and control groups.
Cochrane’s x test (Q test) was used to evaluate the heterogeneity between the studies, and a threshold P value of 0.1 was considered statistically significant. The I2 test was also performed to evaluate the magnitude of heterogeneity between studies.
The pooled estimate of the mean differences in blood pressure (mmHg) between the intervention and control groups was calculated using a fixed-effect model when there was no heterogeneity or a random-effects model (DerSimonian-Laird method) in the presence of significant heterogeneity (I2 > 50%) between the studies.
We performed meta-regression and sensitivity analyses to identify potential sources of heterogeneity in the evaluation of the effects of vitamin D on BP. The covariates used in the meta-regression analyses were chosen by taking into consideration that the variables that seemed to be quite different between the trials, such as different doses of supplements or the age of the patients, could influence the results. Sensitivity analyses were performed to minimize the effects of particular studies that could influence the meta-analysis results. This approach aimed to identify potential outliers by excluding studies such as those with the greatest heterogeneity or the largest sample size.
All statistical analyses were performed using Stata 11.0 software (Stata, College Station, TX, USA). Significance was set at P ≤ 0.05, and 95% confidence intervals are included throughout the paper.
Results
Literature search
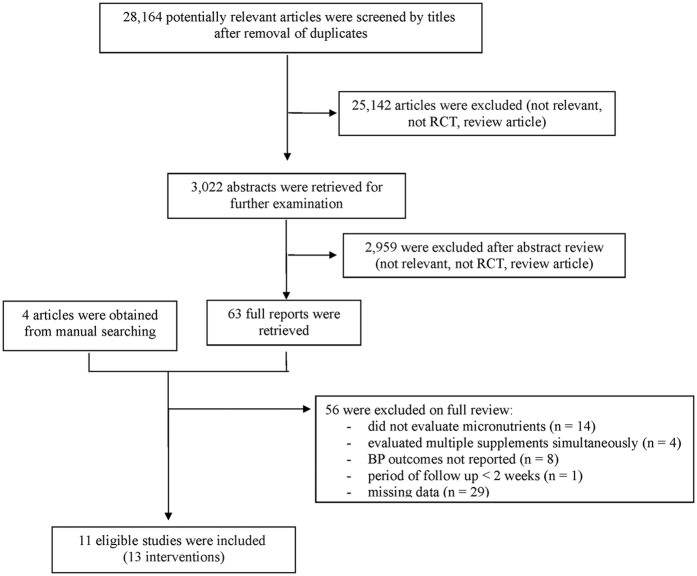
We identified 28,164 articles in our database searches (Fig. 1), and 28,101 articles were excluded based on the title and abstract. We found four articles by manual searching, leaving 67 manuscripts for full-text evaluation. Among those, 56 articles were excluded due to missing relevant data, a design that was not an RCT, BP outcomes that were not reported, or evaluation of the effects of more than one micronutrient at the same time. Therefore, we selected 11 articles15,16,17,18,19,20,21,22,23,24,25 that fulfilled all of the inclusion criteria. Two trials reported two interventions each, so 13 interventions from 11 trials were included in the current systematic review.
Figure 1. Flow diagram of the literature search to identify randomized clinical trials evaluating the effects of micronutrients on blood pressure in patients with type 2 diabetes.
Characteristics of the studies
We divided the RCTs into the following four categories according to the type of micronutrient intervention: sodium, vitamin C, vitamin D, and magnesium. The characteristics of the effects of the included micronutrient interventions on BP in patients with type 2 diabetes are summarized in Table 1. Ten reports were parallel RCTs16,17,18,19,20,21,22,23,24,25, and one was a crossover-controlled trial15. The durations of the trials ranged from 3 to 52 weeks. The included studies provided data from 723 patients with type 2 diabetes and ages ranging from 50.7 to 66.8 years, and 54% of them were males. The duration of diabetes varied from 4.6 to 8.6 years, and six reports18,19,20,23,24,25 did not include this information. The majority of the patients were obese19,20,25 or overweight16,17,18,23, and BMI was not reported in four studies15,21,22,24. Concerning the descriptions of the main characteristics of the intervention and control diets, the dietary intervention was a supplement in most of the reports (10/11), and the intervention in one study was dietary advice only15. The medications in use for diabetes treatment did not differ between the intervention and control groups, and the use of antihypertensive drugs was reported in four studies15,17,19,24. The available data from the reviewed RCTs allowed us to perform meta-analyses for vitamin C and vitamin D supplements.
Table 1. Characteristics of the included studies of the effects of micronutrients on blood pressure in patients with type 2 diabetes.
| Author Year | Design/Trial Duration | Diabetes duration (years) | BMI (kg/m2) | Baseline BP (mmHg) | Diet characteristics and micronutrient measurements | BP change (mean, mmHg) | Medications in use (% of users) | |
|---|---|---|---|---|---|---|---|---|
| Sodium | ||||||||
| Dodson 1989 | Parallel/3 months (1st phase of study) | n = 34 | 4.6 ± 4.3 | NA | SBP | Intervention | Intervention | Oral hypoglycaemic: 18% |
| 67.6% males | I.179.7 ± 18.2 | Advised to restrict daily dietary sodium intake | SBP: −19.2 ± 13.5 | Atenolol: 12% | ||||
| I.61.9 ± 7.5 y | C.173.8 ± 20.3 | Sodium intake* (g/NaCl) | DBP: −3.8 ± 6.9 | |||||
| C.61.1 ± 6.3 y | Baseline: 11.7 g ± 3.9 | |||||||
| Hypertensive subjects | End-of-study: 8.04 ± 2.2 | |||||||
| Change: −3.44 ± 2.48 | ||||||||
| DBP | Control | Control | ||||||
| I.91.4 ± 11.1 | Usual diet for diabetes (ADA) | SBP: −6.2 ± 13.1 | ||||||
| C.92.4 ± 10.9 | Sodium intake* (g/NaCl) | DBP: −2.0 ± 7.2 | ||||||
| Baseline: 10.8 g ± 1.6 | ||||||||
| End-of-study: 10.6 ± 1.9 | ||||||||
| Change: −0.2 ± 1.13 | ||||||||
| Magnesium | ||||||||
| Guerrero Romero 2009 | Parallel/3 weeks | n = 82 | 8.6 ± 0.9 | 29.1 ± 1.3 | SBP | Intervention | Intervention | Glibenclamide: 100% |
| 48.1% males | I.161 ± 26 | Daily oral supplement: 2.5 MgCl (=0.45 g Mg) | SBP: −20.4 ± 15.9 | |||||
| I.58.9 ± 9.0 y | C.154.5 ± 21.2 | Magnesium nmol/l† | DBP: −8.7 ± 16.3 | |||||
| C.60.5 ± 9.4 y | Baseline: 0.62 ± 0.10 | |||||||
| Hypertensive subjects with low serum magnesium and without use of diuretics | End-of-study: 0.81 ± 0.11 | |||||||
| Change: 0.18 ± 0.10 | ||||||||
| DBP | Control/nmol/l† | Control | ||||||
| I.88.4 ± 14.5 | Baseline: 0.61 ± 0.10 | SBP: −4.7 ± 12.7 | ||||||
| C.84.9 ± 12.4 | End-of-study: 0.68 ± 0.11 | DBP: −1.2 ± 12.6 | ||||||
| Change: 0.08 ± 0.14 | ||||||||
| Vitamin C | ||||||||
| Darko 2002 | Parallel/3 weeks | n = 35 | I.9.3 ± 1.2 | 29.1 ± 1.3 | SBP | Intervention | Intervention | Diuretics = 9% |
| 66% males | C.7.8 ± 0.6 | I.141.0 ± 5.0 | Daily oral supplement: 1.5 g ascorbic acid | SBP 0.0 ± 13.4 | ACE inhibitors = 11% | |||
| I.56.6 ± 1.1 y | C.138.0 ± 4.0 | Plasma ascorbate/μmmol | DBP1.0 ± 8.0 | Sulphonylureas = 23% | ||||
| C.55.5 ± 1.8 y | Baseline: 58 ± 6 | Metformin = 37% | ||||||
| End-of-study: 122 ± 10 | ||||||||
| Change: 64 ± 6.5 | ||||||||
| DBP | Control (placebo) | Control | ||||||
| I.80.0 ± 2.0 | Plasma ascorbate/μmmol | SBP: −2.0 ± 10.3 | ||||||
| C.76.0 ± 3.0 | Baseline: 51 ± 5 | DBP: 1.0 ± 7.6 | ||||||
| End-of-study: 53 ± 5 | ||||||||
| Change: 2 ± 3 | ||||||||
| Mullan 2002 | Parallel Double Blind/4 weeks | n = 30 | NA | 28.6 ± 4.3 | SBP | Intervention | Intervention | NA |
| 73% males | I.130.1 ± 12.4 | Daily oral supplement: 500 mg ascorbic acid | SBP: −10.1 ± 7.9 | |||||
| I.57.9 ± 6.6 y | C.129.7 ± 11.7 | Plasma ascorbic acid/μmmol/l | DBP: −4.4 ± 3.7 | |||||
| C.61.0 ± 6.5 y | Baseline: 43.3 ± 19.3 | |||||||
| End-of-study: 78.1 ± 19.5 | ||||||||
| Change: 34.8 ± 12 | ||||||||
| DBP | Control (placebo) | Control: | ||||||
| I.80.5 ± 6.2 | Plasma ascorbic acid: NA | SBP: −1.0 ± 7.4 | ||||||
| C.85.1 ± 6.4 | DBP: −0.6 ± 3.9 | |||||||
| Vitamin D | ||||||||
| Sugden 2008 | Parallel double blind/8 weeks | n = 34 | NA | 31.7 ± 5.4 | SBP | Intervention | Intervention | ACE inhibitor or angiotensin blocker = 62% |
| 53% males | I.145 ± 9.2 | Single dose supplement: 100,000 IU D2 (12,500 IU/week) | SBP: −7.3 ± 11.8 | Metformin = 53% | ||||
| 64.2 ± 9.9 years old plasma vitamin D < 50 mmol/l | C.137 ± 14.1 | Serum 25 OHD/nmol/l | DBP: −2.2 ± 8.6 | Insulin = 18% | ||||
| Baseline: 40.2 ± 10.3 | ||||||||
| Change: 22.9 ± 16.6 | ||||||||
| DBP | Control | Control | ||||||
| I.82 ± 10.5 | Single dose placebo Miglyol® oil | SBP: 6.6 ± 9.7 | ||||||
| C.79 ± 6.0 | Serum 25OHD/nmol/l | DPB: 2.3 ± 5.7 | ||||||
| Baseline: 36.4 ± 8.5 | ||||||||
| Change: 7.6 ± 12.5 | ||||||||
| Witham 2010 | Parallel/16 Weeks | n = 61 | NA | I.31.1 ± 6.7 | SBP | Intervention: 1 | Intervention - 1 | NA |
| 68% males | C.33.3 ± 7.1 | I.149.6 ± 24.8 | Single dose supplement: 100,000 IU D3 (6,250 IU/week) | SBP: −8.2 ± 15.2 | ||||
| I.65.3 ± 9.7 y | C.143.9 ± 24.4 | DBP: −3.6 ± 8.6 | ||||||
| C.66.7 ± 9.7 y | DBP | Serum 25 OHD/nmol/l | ||||||
| I.80.7 ± 14.3 | Baseline: 41 ± 14 | |||||||
| C.80.3 ± 9.7 | End-of-study: 63 ± 20 | |||||||
| Change: 23.0 ± 18.4 | ||||||||
| SBP | Intervention: 2 | Intervention - 2 | ||||||
| I.145.1 ± 25.0 | Single dose supplement: 200,000 IU D3 (12,500 IU/week) | SBP: −5.6 ± 15.7 | ||||||
| C.143.9 ± 24.4 | DBP: −3.1 ± 8.6 | |||||||
| DBP | Serum 25 OHD/nmol/l | |||||||
| I.80.7 ± 14.3 | Baseline: 48 ± 21 | |||||||
| C.80.3 ± 9.7 | End-of-study: 79 ± 31 | |||||||
| Change: 31.0 ± 19.0 | ||||||||
| Control (for interventions 1 and 2) | Control | |||||||
| Single dose placebo Miglyol® oil | SBP: 2.5 ± 14.6 | |||||||
| Serum 25OHD (nmol/l) | DBP: −1.4 ± 6.0 | |||||||
| Baseline: 45 ± 17 | ||||||||
| End-of-study: 54 ± 20 | ||||||||
| Change: 9 ± 12 | ||||||||
| ShabBidar 2011 | Parallel double blind/12 weeks | n = 100 | I.8.3 ± 4.6 | NA | SBD | Intervention | Intervention | Oral antihyperglycemic = 100% (metformin, glibenclamide, glitazones) |
| 43% males | C.7.0 ± 5.2 | I.125.7 ± 14.4 | Vitamin D3-fortified yogurt: 170 mg calcium and 500 IU D3/250 ml, twice/day (total dose 90,000 IU) | SBP: −7.2 ± 12.8 | ||||
| I.52.4 ± 8.4 y | C.128.2 ± 16.6 | DBP: −5.1 ± 6.2 | ||||||
| C.52.6 ± 6.3 y | DBP | Serum 25OHD/nmol/l | ||||||
| I.78.5 ± 10.3 | Baseline: 38.5 ± 20.2 | |||||||
| C.77.8 ± 10.8 | End-of-study: 72 ± 23.5 | |||||||
| Change: 33.5 ± 14.2 | ||||||||
| Control | Control | |||||||
| Plain yogurt: 170 mg calcium without vitamin D3/250ml | SBP: −2.5 ± 11.8 | |||||||
| Serum 25OHD/nmol/l | DBP: −0.8 ± 6.5 | |||||||
| Baseline: 38.5 ± 22.8 | ||||||||
| End-of-study: 33.4 ± 22.8 | ||||||||
| Change: −4.6 ± 14.4 | ||||||||
| Nikooyeh 2011 | Parallel 12 weeks | n = 90 | NA | I.29.9 ± 4.7 | SBP | Intervention (n = 30) | Intervention | Oral antihyperglycemic = 100% (metformin, glibenclamide, glitazones) |
| 39% males | C.29.2 ± 4.4 | I.131.5 ± 21.6 | Vitamin D3-fortified yogurt: 150 mg calcium and 500 IU D3/250 ml, twice/day (total dose 90,000 IU) (7,500 IU/week) | SBP: −3.4 ± 10.3 | ||||
| 50.7 ± 6.1 years old | C.127.3 ± 14.8 | DBP: 0.3 ± 14.0 | ||||||
| DBP | Serum 25OHD/nmol/l | |||||||
| I.77.5 ± 20.0 | Baseline: 44.4 ± 28.7 | |||||||
| C.77.5 ± 10.6 | End-of-study: 77.7 ± 28.6 | |||||||
| Change: 33.3 ± 18.1 | ||||||||
| Control (n = 30) | Control | |||||||
| Plain yogurt: 150 mg calcium without vitamin D3/250 ml | SBP: 0.4 ± 10.0 | |||||||
| Serum 25OHD/nmol/l | DBP: 0.5 ± 7.7 | |||||||
| Baseline: 41.6 ± 44.5 | ||||||||
| End-of-study: 37.2 ± 44.0 | ||||||||
| Change: −4.4 ± 28.0 | ||||||||
| Breslavsky 2013 | Parallel placebo/controlled 52 weeks | n = 47 | NA | NA | SBP | Intervention | Intervention | Metformin = 49% |
| 46.8% males | I.154.2 ± 21.5 | Daily supplement of vitamin D3 1000 mg (total dose 360,000 IU) (6,667 IU/week) | SBP: −10.7 ± 12.8 | Sulfonylurea = 23,4% | ||||
| I.66.8 ± 9.2 y | C.151.8 ± 18.0 | DBP: 0.1 ± 5.3 | ||||||
| C.65.8 ± 9.7 y | DBP | Serum 25OHD/ng/ml | ||||||
| I.76.2 ± 8.8 | Baseline: 11.8 ± 10.9 | |||||||
| C.72.2 ± 10.8 | End-of-study: 17.6 ± 11.5 | |||||||
| Change: 5.8 ± 7.1 | ||||||||
| Control Placebo (microcrystalline cellulose) | Control | Diuretics = 34% | ||||||
| Serum 25OHD/ng/ml | SBP: −11.1 ± 11.4 | ACE inhibitors = 66% | ||||||
| Baseline: 11.7 ± 6.5 | DBP: 2.5 ± 6.6 | B-Blockers = 51% | ||||||
| End-of-study: 14.0 ± 5.9 | ||||||||
| Change: 2.3 ± 3.9 | ||||||||
| Al-Zahari 2013 | Parallel placebo/controlled 12 weeks | n = 200 | NA | I.31.3 ± 4.6 | SBP | Intervention | Intervention | NA |
| 45% males | C.32.0 ± 5.7 | I.123.4 ± 15.8 | 45.000 IU of vitamin D3 every week for 2 months and a single dose of 45000 IU in the last month (total dose 225,000 IU) (18,750 IU/week) | SBP: −1 ± 9.8 | ||||
| I.56.9 ± 9.4 y | C.124.0 ± 15.4 | DBP: −3.2 ± 6.6 | ||||||
| C.52.5 ± 8.1 y | DBP | Serum 25OHD/nmol/l | ||||||
| I.76.4 ± 10.8 | Baseline: 25.3 ± 15.8 | |||||||
| C.75.3 ± 9.2 | End-of-study: 82.8 ± 31.7 | |||||||
| Change: 57.5 ± 21.3 | ||||||||
| Control | Control | |||||||
| Placebo (microcrystalline cellulose) | SBP: 0 ± 9.6 | |||||||
| Serum 25OHD/nmol/l | DBP: −1.6 ± 5.5 | |||||||
| Baseline: 22.0 ± 15.2 | ||||||||
| End-of-study: 55.0 ± 37.5 | ||||||||
| Change: 33 ± 27.2 | ||||||||
| Nasri 2014 | Parallel double blind placebo controlled/12 weeks | n = 60 | NA | NA | SBP | Intervention | Intervention | NA |
| 28.3% males | I.121.0 ± 13.0 | 50,000 IU of vitamin D3 per week (total dose 600,000 IU) | SBP: −11 ± 7.9 | |||||
| 55 ± 10.7 years old | C.118.8 ± 11.0 | DBP: −4.2 ± 4.8 | ||||||
| DBP | Serum 25OHD/nmol/l | |||||||
| I.80.5 ± 8.0 | Baseline: 83.9 ± 52 | |||||||
| C.80.3 ± 7.0 | End-of-study: 164 ± 57 | |||||||
| Change: 80.1 ± 34.8 | ||||||||
| Control | Control | |||||||
| Serum 25OHD/nmol/l | SBP: −4.2 ± 6.6 | |||||||
| Baseline: 105.7 ± 64 | DBP: −1.3 ± 4.5 | |||||||
| End-of-study: 115.8 ± 94 | ||||||||
| Change: 10.1 ± 57.5 | ||||||||
Abbreviations: ADA = American Diabetes Association; BP = blood pressure; C = control group; DBP = diastolic blood pressure; DM = diabetes mellitus; I = intervention group; NA = not available; SBP = systolic blood pressure; y = years old; *NaCl intake estimated by 24-h urinary sodium (g), †magnesium intake based on 24-h urinary magnesium (mmol/L).
Data are expressed as the mean (standard deviation).
Summary of Evidence
Sodium
Only one study15 including 34 hypertensive type 2 diabetic patients with a mean age of 61.4 years (67.6% males) evaluated the effects of sodium on BP. In the first phase of that study, 34 patients were randomly advised to reduce their daily sodium intake for 3 months. A significant reduction of approximately 20 mmHg in supine systolic BP was observed, which was associated with a reduction in daily salt intake of approximately 3 g (corresponding to a daily salt consumption of 11.6 to 8.2 g). The patients who were part of the salt intake reduction arm were included in a one-month crossover-randomized trial to evaluate the effects of sodium supplementation. It was unclear if there was a washout period between the 1st and 2nd phases, and only nine patients completed this study.
Magnesium
We identified only one RCT16 that examined the effects of magnesium supplementation on BP over three weeks in 82 type 2 diabetic patients with hypertension and hypomagnesaemia (mean 0.62 mmol/l; reference range 0.70 to 0.85 mmol/l). In this double-blind, placebo-controlled RCT, the participants did not have chronic diarrhoea, heavy alcohol intake, use of diuretics and/or calcium antagonist drugs, previous oral magnesium supplementation, or ischaemic or renal diseases. Daily supplementation of 450 mg of magnesium reduced both SBP (−20.4 mmHg) and DBP (−8.7 mmHg), and the risk of bias in that trial was low for most of the evaluated domains (Table 2).
Table 2. Assessment of the quality of the studies included in the systematic review: a summary of risk of bias (Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0).
| Selection Bias |
Performance Bias | Detection Bias | Attrition Bias | Reporting Bias | Other Bias | ||
|---|---|---|---|---|---|---|---|
| Random sequence generation | Allocation concealment | Blinding of participant and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Diet/supplement compliance assessment | |
| Sodium | |||||||
| Dodson15 | unclear | unclear | low* | low | low | low | low |
| Magnesium | |||||||
| Guerrero-Romero16 | low | unclear | low | unclear | low | low | low |
| Vitamin C | |||||||
| Darko17 | unclear | unclear | low | unclear | low | low | low |
| Mullan18 | unclear | unclear | low | unclear | low | low | low |
| Vitamin D | |||||||
| Witham19 | low | low | low | unclear | low | low | low |
| Sugden20 | low | low | low | unclear | low | low | low |
| Shab-Bidar21 | unclear | unclear | low | unclear | low | low | low |
| Nasri22 | low | low | low | unclear | low | low | low |
| Al-Zahrani | low | low | high | unclear | high | unclear | unclear |
| Nikooeh | unclear | unclear | unclear | unclear | unclear | unclear | low |
| Breslavsky | unclear | unclear | low | unclear | unclear | low | unclear |
*Blinding of participants and personnel was not applicable because the intervention was dietary advice only.
Vitamin C
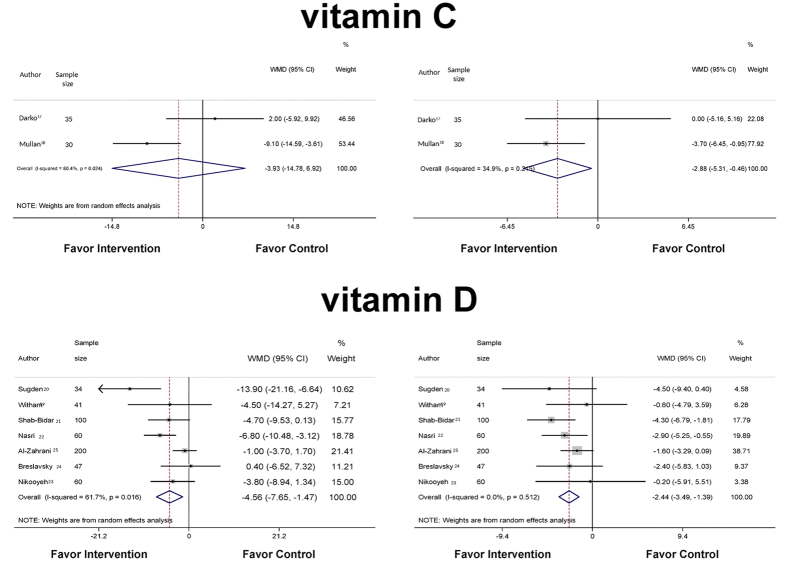
A total of 65 patients, 69% male, with ages ranging from 51.8 to 66.5 years old and with a mean BMI of 28.9 kg/m2, were evaluated in two studies17,18 examining the effects of vitamin C supplements (500 mg to 1,500 mg/day) on BP. When a meta-analysis was performed (Fig. 2), no effect of vitamin C on systolic BP was observed (WMD −3.93 mmHg; 95%CI −14.78, 6.92; P = 0.478). However, a reduction of −2.88 mmHg (WMD; 95%CI −5.31, −0.46; P = 0.020) in diastolic BP was observed compared with the control groups (Fig. 2). High heterogeneity in the systolic BP (I2 80.4%; P = 0.024) but not in the diastolic BP (I2 34.9%; P = 0.215) was observed between the studies.
Figure 2. Forest plots of the effects of vitamin C and vitamin D on the blood pressure of patients with type 2 diabetes.
Vitamin D
Seven studies (eight interventions)19,20,21,22,23,24,25 involving 542 patients with type 2 diabetes, 47% male, with ages ranging from 50.7 to 66.8 years, evaluated the effects of vitamin D on BP. Three of the studies21,23,24 did not include BMI values, and antihypertensive medication was described in only two of the RCTs20,24. Seven of the trials evaluated two different forms of vitamin D19,20,21,22,23,24,25. One study20 evaluated supplementation of ergocalciferol (vitamin D2), and six trials19,21,22,23,24,25 evaluated cholecalciferol (vitamin D3). In three of the interventions, the patients received a single dose of vitamin D220 (100,000 IU) or vitamin D319 (100,000 IU or 200,000 IU). One trial evaluated the effects of 50,000 IU/week of cholecalciferol for 12 weeks (a total dose of 600,000 IU)22, and the other study evaluated the effects of 225,000 IU of vitamin D3 (45,000 IU/week for 2 months and a single dose of 45,000 IU in the last month)24. One study25 evaluated the effects of daily supplementation with vitamin D3 by administering 1000 IU/day for 12 months, and two trials evaluated the effects of vitamin D3-fortified yogurt21,23 (500 IU D3 in 250 ml twice a day for 12 weeks; a total dose of 90,000 IU).
We pooled data from seven studies19,20,21,22,23,24,25 (Fig. 2), and the study by Witham et al.19 used the same control group for all tested dosages. Therefore, we included the data for only one vitamin D dosage (100,000 IU) to prevent the same individuals from being inappropriately included twice in the pooled estimate and because this dose was similar to doses used in other studies20,21,23. Our meta-analysis demonstrated that supplementation of vitamin D led to a 4.6 mmHg decrease (WMD; 95%CI −7.65, −1.47; P = 0.004; I2 61.7%; P = 0.016) in systolic BP compared with the control group. The diastolic BP also decreased after intervention [WMD −2.44 mmHg (95%CI −3.49, −1.39; P < 0.001); I2 0%; P = 0.512]. When the meta-analysis was performed using the data from the 200,000 IU arm instead of the 100,000 IU arm of the Witham et al. study19, the significance of the reductions in systolic BP (WMD −4.600 mmHg; 95%CI −7.714, −1.485, P = 0.004; I2 61.8%, P = 0.015) and diastolic BP (WMD −2.398 mmHg; 95%CI −3.611, −1.186; P < 0.001; I2 14.7%, P = 0.318) did not change.
Patients from the study by Shab-Bidar et al.21 along with patients from the study by Nasri et al.22 seemed to be younger than the patients from other studies. Additionally, the weekly vitamin dose used by Nasri et al.22 was higher than the doses used in the other studies, and the durations of follow-up of the studies were quite different (8 to 52 weeks). Therefore, our meta-regression analysis included vitamin D doses (90,000; 100,000; 225,000; 360,000; 600,000 IU) and age as covariates. Neither age, duration of follow-up nor doses were associated with changes in systolic BP (P = 0.482) or diastolic BP (P = 0.693). When we included the calculated daily dose of vitamin D instead of the total vitamin dose in the same model, there were also no associations between the doses and the changes in both systolic BP (P = 0.855) and diastolic BP (P = 0.684).
We performed two sensitivity analyses. In the first one, we excluded the study with the highest weighted mean difference [47.50% in SBP and 37.52% in DBP; ref. 21], and the significant reduction in BP remained for both systolic BP (WMD −7,081 mmHg; 95%CI −10.798, −3.365, P < 0.001; I2 56.4%, P = 0.101) and diastolic BP (WMD −3.521 mmHg; 95%CI −5.482, −1.559, P = 0.002; I2 16.5%, P = 0.302). In the second sensitivity analysis, the study with the most heterogeneity25 was excluded, and the significant reduction in BP also remained for both systolic BP (WMD −5,529 mmHg; 95%CI −8.679, −2.379, P < 0.001; I2 44.2%, P = 0.110) and diastolic BP (WMD −2.965 mmHg; 95%CI −4.306, −1.624; P < 0.001; I2 0.0%, P = 0.592).
The quality of the body of evidence in our systematic review, specifically for the studies included in the meta-analyses performed according to the GRADE approach, was considered moderate for vitamin D and low for vitamin C (Table 3).
Table 3. GRADE evidence profiles for the meta-analysis of the effects of vitamin C and vitamin D on blood pressure in patients with type 2 diabetes.
| Vitamin C | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessment |
Summary of Findings |
|||||||||
| Participants (studies) Follow-up | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Overall quality of evidence | Study event rates (%) |
Relative effect (95% CI) | Anticipated absolute effects | |
| Intervention | Control | |||||||||
| Systolic Blood Pressure (IMPORTANT OUTCOME; Better indicated by lower values) | ||||||||||
| 65 (2 studies) 3.5 weeks | Serious1 | Serious2 | not serious | Serious3 | undetected |
    VERY LOW due to risk of bias, inconsistency, imprecision
VERY LOW due to risk of bias, inconsistency, imprecision |
32 | 33 | — | The mean systolic blood pressure in the intervention group was 3.93 lower (14.78 lower to 6.92 higher) |
| Diastolic Blood Pressure (IMPORTANT OUTCOME; Better indicated by lower values) | ||||||||||
| 65 (2 studies) 6.35 weeks | serious1 | serious2 | no serious indirectness | no serious imprecision | undetected |
    LOW1,2 due to risk of bias, inconsistency
LOW1,2 due to risk of bias, inconsistency |
32 | 33 | — | The mean diastolic blood pressure in the intervention group was 2.88 lower (2.88 to 0.46 lower) |
| Vitamin D | ||||||||||
| Systolic Blood Pressure (Better indicated by lower values) | ||||||||||
| 542 (7 studies) 3–52 weeks | no serious risk of bias | Serious2 | no serious indirectness | no serious imprecision | undetected |
    MODERATE due to inconsistency
MODERATE due to inconsistency |
272 | 270 | — | The mean systolic blood pressure in the intervention group was 4.558 lower (7.65 to 1.465 lower) |
| Diastolic Blood Pressure (IMPORTANT OUTCOME; Better indicated by lower values) | ||||||||||
| 542 (7 studies) 3–52 weeks | no serious risk of bias | no serious inconsistency | no serious indirectness | no serious imprecision | undetected |
    HIGH
HIGH
|
272 | 270 | — | The mean diastolic blood pressure in the intervention group was 2.437 lower (3.487 to 1.387 lower) |
1Most of the information concerning randomization and blinding was unclear in the analyses of the individual trials.
2High heterogeneity.
3Few patients analysed.
Discussion
In this systematic review of the effects of micronutrients on BP in patients with type 2 diabetes, we evaluated the effects of vitamin C, vitamin D, sodium, and magnesium. In pooled analyses, we demonstrated that supplemental vitamin C reduces diastolic BP and vitamin D reduces both systolic and diastolic BP.
Vitamin C
A previous meta-analysis that evaluated 29 RCTs31 has already demonstrated a beneficial effect of supplemental vitamin C on BP. In that study, only five of the 29 included RCTs involved patients with diabetes, and in three of them, the supplemented vitamin C was combined with other micronutrients. In our meta-analysis, only patients with diabetes were included, and the individual effect of vitamin C on diastolic BP was demonstrated. However, the low quality of the included studies, especially the small number of studied patients, should be taken into in account. Therefore, we believe that the effects of vitamin C on BP in patients with type 2 diabetes remain unclear.
Vitamin D
In the present meta-analysis of the effect of vitamin D on BP in patients with type 2 diabetes, we demonstrated a reduction in BP, especially in systolic BP. In all of the evaluated studies, the supplemented vitamin D was either ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3). Although these vitamins are derived from different sources (plants, diet, or dermal synthesis), both require enzymatic conversion in the liver and kidney to active metabolites, and they were orally administered in all studies. Most importantly, they have the same expected biological effects32.
We identified three other meta-analyses of the effects of vitamin D on BP33,34,35. The first meta-analysis33 demonstrated a reduction in DBP of 3.1 mmHg in hypertensive patients. Of the 11 included RCTs, only one trial involved patients with diabetes. Moreover, the age range of the patients (48 to 74 years), the duration of the interventions (5 to 52 weeks), and the use of active (1–25 OHD) or inactive (vitamin D2, D3, UVB radiation) forms of vitamin D were associated with high heterogeneity of BP effects among the RCTs. Recently, Lee et al.35 have also demonstrated a small reduction in diastolic BP due to vitamin D supplementation in patients with type 2 diabetes. On the other hand, the meta-analyses of Beveridge et al.34 demonstrated that vitamin D did not reduce BP in the general population and in a subgroup analysis of 353 patients with diabetes. In contrast to our analysis, both of these meta-analyses34,35 included studies that examined the effects of paricalcitol, as well as intramuscular and parenteral vitamin D, on BP. Moreover, the results of studies in which BP was measured by office or ambulatory monitoring were evaluated together in the same meta-analysis34,35. Unlike our analysis, these meta-analyses33,34,35 did not include the studies of Nasri et al.22 and Al-Zahari et al.25.
In the current vitamin D meta-analysis, a moderate heterogeneity of effect was observed between RCTs. In addition, when we excluded the study21 that contributed the largest reduction in pooled BPs or the study with the greatest heterogeneity25, the results did not change. Moreover, it would be of interest to know if the effects of vitamin D on BP occur mainly in patients with hypovitaminosis. Most of the studies19,20,21,22,23,24,25 included patients who had serum concentrations of vitamin D that were lower than 50 nmol/l, and this cut-off value has been considered to be diagnostic of a vitamin D deficiency36. However, we cannot make generalizations concerning the observed beneficial effects of vitamin D on BP in patients with sufficient levels of vitamin D. Although most of the studies included in our meta-analysis had a low risk of bias, the quality of the body of evidence in the current vitamin D meta-analysis was classified as moderate29. This means that additional studies are still desirable to confirm our results.
Observational and experimental data favour the concept that vitamin D is associated with the pathogenesis of arterial hypertension37,38. A possible mechanism for this link involves the inhibition of the rennin-angiotensin-aldosterone system by vitamin D. Additionally, in the presence of hypovitaminosis D, an alternative mechanism could be related to the secondary hyperparathyroidism and relative hypocalcemia that are commonly seen in these patients38.
Sodium
The effects of the reduction of sodium intake on BP, particularly in patients with diabetes, were described in only one study published at the end of the 1980s15. Although salt restriction in hypertensive patients with or without diabetes is highly recommended6,9,11, the salt restriction phase of that study was short. Moreover, that trial involved a small sample of patients. Therefore, the specific effects of sodium restriction on BP in type 2 diabetic patients could not be properly evaluated.
Magnesium
Concerning magnesium supplementation, only one study described a reduction in BP in diabetic patients16. The absence of side effects of magnesium supplementation in this trial (e.g., nausea, vomiting, hypocalcaemia, or even cardiovascular effects) was probably due to the presence of hypomagnesaemia and the use of a magnesium dose that was similar to the recommendations for daily intake32. Even though that study was well conducted with almost no bias, we believe that their results are not applicable to type 2 diabetic patients who do not have a magnesium deficiency.
Limitations and strengths of our study
One limitation of our systematic reviews and meta-analyses was the inclusion of few reports, despite the large number of revised trials. However, because the evaluated data may comprise the currently available literature on this topic, we believe that our study provides important information that adds to the current knowledge of the impact of micronutrients on BP in patients with diabetes. Another possible limitation is that the BPs were evaluated only in the office in all of the included RCTs. Office BP measurements comprise the usual assessment in clinical practice, but a more comprehensive evaluation of BP homeostasis could be obtained by continuous BP monitoring38. Finally, we could not find any long-term RCTs of the effects of micronutrients on BP in patients with diabetes, as well as analyses of hard outcomes such as mortality.
The present systematic review and meta-analyses were conducted in accordance with the Cochrane26,28 and PRISMA27 guidelines. A very extensive literature search strategy was used, regardless of language, that included both micronutrient and macronutrient terms. In addition, we excluded studies that evaluated more than one micronutrient intervention together or any combined nutritional interventions in order to assess the actual effect of the micronutrient on BP.
Conclusion
In conclusion, our systematic review and meta-analyses of RCTs demonstrated that the supplementation of vitamin D may reduce both systolic and diastolic BP in patients with type 2 diabetes. The effect of vitamin C, although significant, was not definitively demonstrated. Finally, our data suggest that these interventions could be adopted to treat high BP in patients with diabetes, especially in the presence of hypovitaminosis D. However, further studies with long-term follow-up periods and large samples of hypertensive type 2 diabetic patients are needed.
Additional Information
How to cite this article: de Paula, T. P. et al. Effect of individual micronutrients on blood pressure in patients with type 2 diabetes: systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 7, 40751; doi: 10.1038/srep40751 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
This study was supported by grants from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundo de Incentivo à Pesquisa (FIPE) of Hospital de Clínicas de Porto Alegre (HCPA). T.P.P. received grants from Projeto Nacional de Pós-Doutorado (PNPD/CAPES).
Footnotes
Author Contributions Study concept and design: M.J.A., C.K.K.; researched data: T.P.P., C.K.K.; analysis and interpretation of data: T.P.P., C.K.K., L.V.V., M.J.A.; drafting of the manuscript: T.P.P., C.K.K., M.J.A.; and critical revision of the manuscript for important intellectual content: T.P.P., C.K.K., L.V.V., M.J.A.
References
- Stratton I. M. et al. Additive effects of glycaemia and blood pressure exposure on risk of complications in type 2 diabetes: a prospective observational study (UKPDS 75). Diabetologia 49, 1761–1769 (2006). [DOI] [PubMed] [Google Scholar]
- American Diabetes Association Diabetes Statistics. Internet: http://www.diabetes.org/diabetes-basics/diabetes-statistics/?loc=DropDownDB-stats 2011; Date of acess 17/01/2016 (2016).
- Pinto L. C. et al. Inadequate blood pressure control in patients with type 2 diabetes mellitus. Arq Bras Cardiol 94, 651–655 (2010). [DOI] [PubMed] [Google Scholar]
- James P. A. et al. Evidence-Based Guideline for the Management of High Blood Pressure in Adults Report from the Panel Members Appointed to the Eighth Joint National Committee (JNC 8). JAMA 311(5), 507–520 (2014). [DOI] [PubMed] [Google Scholar]
- Heerspink H. J. L. et al. Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int 82, 330–337 (2012). [DOI] [PubMed] [Google Scholar]
- Sacks F. M. & Campos H. Dietary therapy in hypertension. N Engl J Med 362, 2102–2112 (2010). [DOI] [PubMed] [Google Scholar]
- Appel L. J. et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N Engl J Med 336, 1117–1124 (1997). [DOI] [PubMed] [Google Scholar]
- Sacks F. M. et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. N Engl J Med 344, 3–10 (2001). [DOI] [PubMed] [Google Scholar]
- Standards of medical care in diabetes DiabetesCare 39 (Suppl 1), S1–S110 (2016). [Google Scholar]
- National Institute for Clinical Excellence. CG127 hypertension clinical management of primary hypertension in adults. NICE Guideline (2011). [Google Scholar]
- Mancia G. et al. ESH/ESC Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). European Heart Journal 34, 2159–2219 (2013). [DOI] [PubMed] [Google Scholar]
- Paula T. P. et al. The role of dietary approaches to stop hypertension (DASH) diet food groups in blood pressure in type 2 diabetes. Br J Nutr 108, 155–162 (2012). [DOI] [PubMed] [Google Scholar]
- Azadbakht L. et al. Effects of the dietary approaches to stop hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients. Diabetes Care 34, 55–57 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula T. P. et al. Effects of the DASH Diet and Walking on Blood Pressure in Patients With Type 2 Diabetes and Uncontrolled Hypertension: A Randomized Controlled Trial. J Clin Hypertens 17, 895–901 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson P. M. et al. Sodium restriction and blood pressure in hypertensive type II diabetics: randomized blind controlled and crossover studies of moderate sodium restriction and sodium supplementation. BMJ 298, 227–30 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero-Romero M. & Rodrigues Moran M. The effect of lowering blood pressure by magnesium supplementation in diabetic hypertensive adults with low serum magnesium levels: a randomized, doubleblind, placebo-controlled clinical trial. J Hum Hypertens 23, 245–251 (2009). [DOI] [PubMed] [Google Scholar]
- Darko D. et al. Lack of effect of oral vitamin C on blood pressure, oxidative stress and endothelial function in type II diabetes. ClinSci ( lond) 103, 339–344 (2002). [DOI] [PubMed] [Google Scholar]
- Mullan B., Young I. S., Fee H. & Mccance D. Ascorbic acid reduces blood pressure and arterial stiffness in type 2 diabetes. Hypertension 40, 804–809 (2002). [DOI] [PubMed] [Google Scholar]
- Witham M. D. et al. The effect of different doses of vitamin D3 on markers of vascular health in patients with type 2 diabetes: a randomised controlled Trial. Diabetologia 53, 2112–2119 (2010). [DOI] [PubMed] [Google Scholar]
- Sugden J. A. et al. Vitamin D improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diabet Med 25, 320–325 (2008). [DOI] [PubMed] [Google Scholar]
- Shab-Bidar S. et al. Regular consumption of vitamin D-fortified yogurt drink (Doogh) improved endothelial biomarkers in subjects with type 2 diabetes: a randomized double-blind clinical trial. BMC Medicine 9, 125–135 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasri H. et al. Impact of oral vitamin D (cholecalciferol) replacement therapy on blood pressure in type 2 diabetes patients; a randomized, double-blind, placebo controlled clinical trial. J Nephropathol 3, 29–33 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikooyeh B., Neyestani T. R., Farvid M. et al. Daily consumption of vitamin D− or vitamin D+ calcium-fortified yogurt drink improved glycemic control in patients with type 2 diabetes: a randomized clinical trial. Am J Clin Nutr 93, 764–71 (2011). [DOI] [PubMed] [Google Scholar]
- Breslavsky A., Frand J., Matas Z. et al. Effect of high doses of vitamin D on arterial properties, adiponectin, leptin and glucose homeostasis in type 2 diabetic patients. Clin Nutr 32, 970–975 (2013). [DOI] [PubMed] [Google Scholar]
- Al-Zahrani et al. A 3-month oral vitamin D supplementation marginally improves diastolic blood pressure in Saudi patients with type 2 diabetes mellitus. Int J Clin Exp Med 7, 5421–5428 (2014). [PMC free article] [PubMed] [Google Scholar]
- Higgins J. P. T. & Green S. (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from http://http://handbook.cochrane.org/ Date of access: 29/09/2016 (2011).
- Liberati A. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62, 1–34 (2009). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balshem H. et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64, 401–406 (2011). [DOI] [PubMed] [Google Scholar]
- Follmann D. et al. Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 45, 769–773 (1992). [DOI] [PubMed] [Google Scholar]
- Juraschek S. P. et al. Effects of vitamin C supplementation on blood pressure: a meta-analysis of randomized controlled trials. Am J Clin Nutr 95, 1079–1088 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride. Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Washington (DC): National Academies Press (US); ISBN-10: 0-309-06350-7ISBN-10: 0-309-06403-1 (1997). [PubMed]
- Witham M. D., Adnan Nadir M. & Struthers A. D. Effect of vitamin D on blood pressure: a systematic review and meta-analysis. J Hypertension 27, 1948–1954 (2009). [DOI] [PubMed] [Google Scholar]
- Beveridge L. A. et al. Effect of Vitamin D Supplementation on Blood Pressure A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA 175(5), 745–754 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. J. & Lee Y. J. Effects of vitamin D on blood pressure in patients with type 2 diabetes mellitus. Int J Clin Pharm and Therap 4, 233–242 (2016). [DOI] [PubMed] [Google Scholar]
- Holick M. F., Bischoff-Ferrari H. A., Gordon C. M. et al. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice Guideline. J Clin Endocrin Metab 96, 1911–1930 (2011). [DOI] [PubMed] [Google Scholar]
- McGreevy C. & Williams D. New insights about vitamin D and cardiovascular disease. Ann Intern Med 155, 820–882 (2011). [DOI] [PubMed] [Google Scholar]
- Grossman H. Ambulatory blood pressure monitoring in the diagnosis and management of hypertension. Diabetes Care 36(suppl 2), 307–331 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.