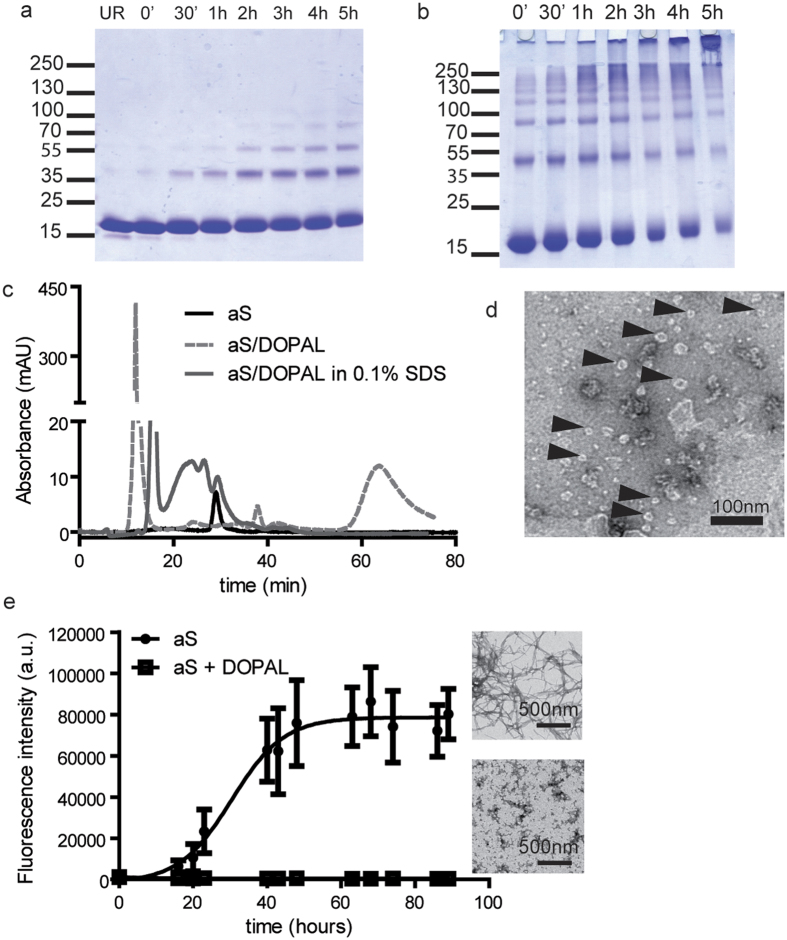
Figure 5. Biophysical and biochemical characterization of aS-DOPAL monomer and oligomers.
(a) SDS-PAGE of samples from the aS and DOPAL reaction performed at 25 °C for 5 hours collected at different time points. The reaction products were obtained reacting 67 μM aS in a stoichiometric ratio of 1:20 with DOPAL. (b) SDS-PAGE showing the reaction products of 310 μM aS in a stoichiometric ratio of 1:5 with DOPAL. (c) Size-exclusion chromatography of monomeric aS compared to the reaction products of 67 μM aS in a stoichiometric ratio of 1:20 with DOPAL, which are large oligomers eluting in the void volume of the column. The graph also shows the chromatographic profile of aS-DOPAL oligomers in the presence of 0.1% SDS. The elution profile of aS-DOPAL oligomers changes in the presence of SDS and shows peaks at lower molecular weights further confirming that SDS can disaggregate the aS-DOPAL non-covalent oligomers into the ensemble of dimers, trimers and tetramers reported in panel a. (d) TEM image of aS-DOPAL oligomers showing annular shapes. (e) ThT kinetic of aS aggregation alone (full circles) shows the typical sigmoidal shape and lead to the formation of canonical amyloid fibrils. The aggregation process of aS-DOPAL molecules (empty squares) shows that they do not acquire a beta-sheet structure and the final products of the aggregation are not fibrils but amorphous aggregates.