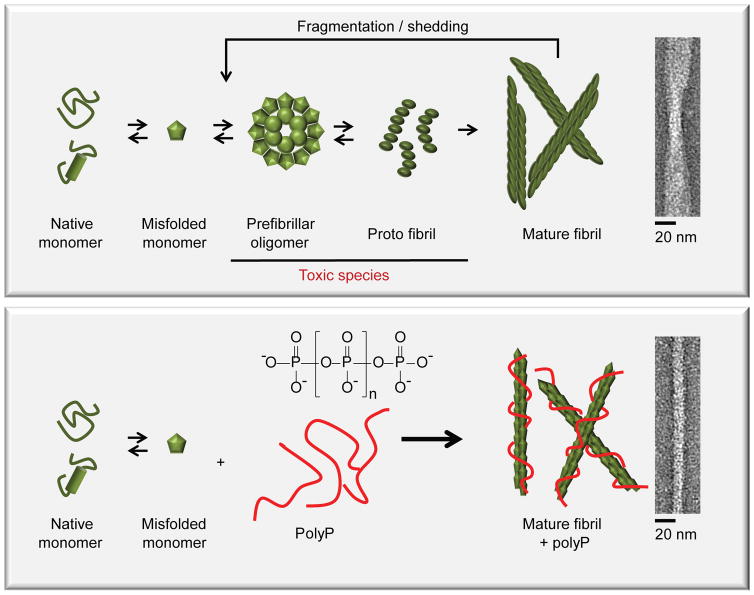
Figure 6. PolyP accelerates fibril formation and increases fibril stability.
Alpha-helical or natively unfolded amyloid monomers undergo structural rearrangements, leading to the formation of β-sheet rich oligomers and protofibrils, which are considered to be the primary cytotoxic species. Further assembly leads to the formation of cross-β-sheet fibrils, which are thought to serve as reservoirs for toxic intermediates as they disassemble back into oligomeric species in a process termed shedding. PolyP dramatically accelerates initial fibril formation, and increases fibril stability, thereby reducing the formation of toxic oligomeric species. This mechanism likely explains how presence of polyP mitigates amyloid cytotoxicity.