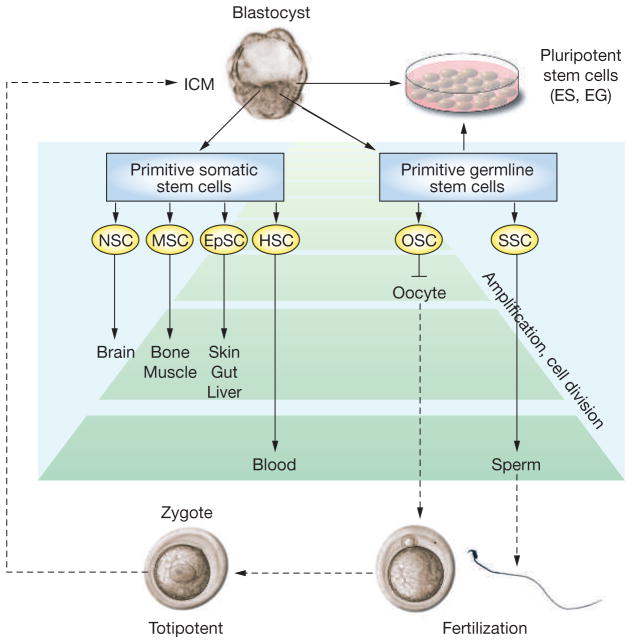
Figure 1.
Lineage development of somatic and germline stem cells in vivo and pluripotent stem cells in vitro. Inner-cell-mass cells of the blastocyst generate all somatic and germ cells of the fetus. Primitive fetal somatic stem cells develop into adult somatic tissue-specific stem cells. Primitive germline stem cells, which are called primordial germ cells, generate male or female germ cells depending on the sex of the gonad. In the fetal ovary, oogonial stem cells cease proliferation before birth and enter the first meiotic division; therefore, they have no stem-cell potential after birth. In the fetal testis, primordial germ cells develop into gonocytes, which soon enter mitotic arrest; however, they become spermatogonial stem cells after birth and self-renew as well as produce daughter cells that commit to differentiate into spermatozoa throughout life. During spermatogenesis, one spermatogonium undergoes up to 12 cell divisions before forming mature spermatozoa.8,15 The number of amplification cell divisions of spermatogonial stem cells and hematopoietic stem cells is greater than for other adult stem cells, and they are considered the most productive adult stem-cell systems.8 Pluripotent stem cells, embryonic stem cells and embryonic germ cells, can be derived in vitro from inner-cell-mass or primordial germ cells, respectively. The developmental potential of these pluripotent stem cells, when transplanted to a blastocyst, is similar to epiblast cells in vivo. Embryonic stem cells and embryonic germ cells self-renew in vitro under appropriate culture conditions.
EG cell, embryonic germ cell; EpSC, epithelial stem cell; ES cell, embryonic stem cell; HSC, hematopoietic stem cell; ICM, inner cell mass; MSC, mesenchymal stem cell; NSC, neuronal stem cell; OSC, oogonial stem cell; SSC, spermatogonial stem cell.