Scheme 1. General Synthetic Protocol Utilized To Access the Protected and Deprotected Truncated Autoinducing Peptide Analoguesa.
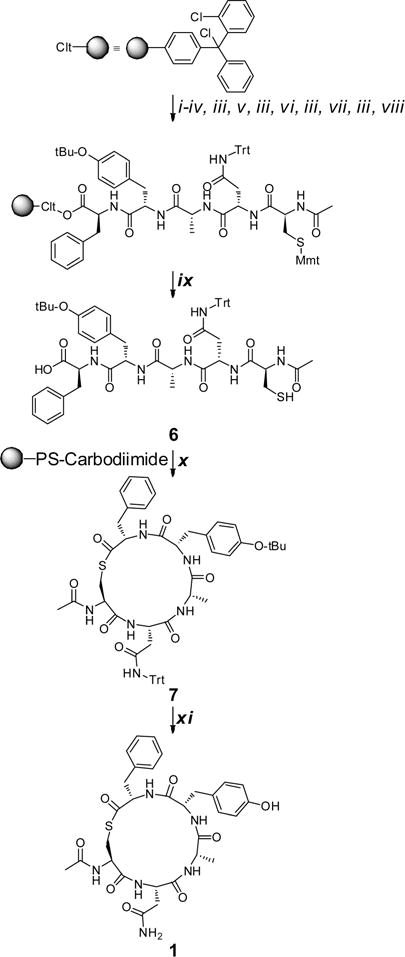
aReagents and conditions: (i) Fmoc-Phe-OH (4 equiv), DIPEA (8 equiv), DCM, rt, 2 h; (ii) MeOH, rt, 20 min; (iii) 20% piperidine/DMF; (iv) Fmoc-Tyr(O-t-Bu)-OH (4 equiv), HCTU (4 equiv), DIPEA (8 equiv), DMF, 40 °C, 1 h; (v) Fmoc-Ala-OH (4 equiv), HCTU (4 equiv), DIPEA (8 equiv), DMF, 40 °C, 1 h; (vi) Fmoc-Asn(Trt)-OH (4 equiv), HCTU (4 equiv), DIPEA (8 equiv), DMF, 40 °C, 1 h; (vii) Fmoc-Cys(Mmt)-OH (4 equiv), HCTU (4 equiv), DIPEA (8 equiv), DMF, 40 °C, 1 h; (viii) acetic anhydride/DIPEA (1:2), rt, 0.5 h; (ix) DCM/TFA/Et3SiH (96:2:2); (x) N′-polystyrene methyl-N′-cyclohexylcarbodiimide (3 equiv), CHCl3, rt; (xi) TFA/Et3SiH/H2O (95:2.5:2.5), rt, 6 h.
