Abstract
Background
Improved detection and linkage to care of previously undiagnosed HIV infections requires innovative approaches to testing. We sought to determine the feasibility of targeted HIV testing in geographic areas, defined by continuum of care parameters, to identify HIV-infected persons needing linkage or engagement in care.
Methods
Using HIV surveillance data from Washington, DC, we identified census tracts (CTs) that had an HIV prevalence >1% and were either above (higher risk areas--HRAs) or below (lower risk areas--LRAs) the median for three indicators: monitored viral load, proportion of persons out of care (OOC) and never in care. Community-based HIV rapid testing and participant surveys were conducted in the twenty CTs meeting the criteria. Areas were mapped using ArcGIS and descriptive and univariate analyses were conducted comparing the areas and participants.
Results
Among 1,471 persons tested, 28 (1.9%) tested HIV-positive; 2.1% in HRAs vs. 1.7% in LRAs (p=0.57). Higher proportions of males (63.7% vs. 56.7%, p=0.007) and fewer blacks (91.0% vs. 94.6%, p=0.008) were tested in LRAs vs. HRAs; no differences were observed in risk behaviors between the areas. Among HIV-positive participants, 54% were new diagnoses (n=9) or OOC (n=6), all were black, 64% were male with a median age of 51 years.
Conclusions
While significant differences in HIV seropositivity were not observed between testing areas, our approach proved feasible and enabled identification of new diagnoses and OOC HIV-infected persons. This testing paradigm could be adapted in other locales to identify areas for targeted HIV testing and other re-engagement efforts.
Keywords: HIV care continuum, surveillance, HIV testing, geographic distribution, targeted testing
Background
A key component of the United States National HIV/AIDS strategy (NHAS) is to reduce the number of new HIV infections by increasing the detection of undiagnosed HIV infections [1]. The 2020 updated NHAS also calls specifically for emphasis on “widespread” HIV testing and linkage to care and support for people to remain engaged in HIV care [2]. Similarly, the UNAIDS 90-90-90 goals call for 90% of HIV-infected persons to be aware of their serostatus, on ART, and virally suppressed [3]. In response to these goals, a combination of strategies including routine HIV testing and treatment as prevention are focused on identifying persons who are undiagnosed and linking them to care and treatment, with the aim of preventing new infections. HIV testing, in particular, is of paramount importance as studies have shown that persons who are HIV infected but unaware of their infections and those who are diagnosed but not in care account for 91.5% of new transmissions [4]. Moreover, once diagnosed, such individuals are likely to reduce their risky behaviors thereby reducing their risk of infecting others [5].
In order for the NHAS and 90-90-90 goals to be successful, innovative approaches to efficiently and effectively find and test previously undiagnosed persons and link them to care are necessary. A variety of approaches have proven successful in understanding where infections are occurring. Geospatial and geovisualization approaches have included cluster analyses to define the relationship between HIV and community-level factors [6, 7], descriptions of the spatial distribution of high risk behaviors and high HIV infection rates [7–10], and identification of areas for tuberculosis, syphilis and HIV screening [11].
The geographic distribution of HIV has also been described using routinely collected public health HIV surveillance data to map HIV prevalence areas, to measure community viral load (CVL), to direct research and testing, and to assess the impact of HIV testing efforts. Mapping of HIV prevalence is routinely conducted at the state and local level to understand the distribution of disease burden and to guide testing for studies such as National HIV Behavioral Surveillance (NHBS) [12]. More recently, mapping to visualize the HIV care continuum and related sociodemographic factors has been done in an effort to understand how geographic factors influence the continuum [6, 13, 14]. Additionally, mapping of surveillance data has also been used to estimate CVL, a measure of the potential infectivity of a community [15]. Declines in CVL have been shown to be directly correlated with a decline in new HIV diagnoses [16–21] and in combination with geospatial analyses, CVL has been used to successfully identify concentrated areas of high HIV prevalence or “hot spots” where prevention, care, and treatment interventions are needed [16–19, 22].
In Washington, DC, a city with a generalized HIV epidemic (2.4% prevalence), many of these aforementioned approaches have been used to address the epidemic. Significant declines in new HIV diagnoses over recent years have been achieved concurrent with the widespread scale-up of routine HIV testing, comprehensive linkage and retention in care programs, and universal access to care [23–26]; however, measurement of care-continuum outcomes and CVL continue to identify gaps in meeting the NHAS and UNAIDS 90-90-90 goals. The DC HIV care continuum suggests that as many as 25–30% of infected people are unaware of their infections [27–29]. Furthermore, despite linkage to care rates of approximately 80%, only 40% of persons were continuously in care, and 47% achieved viral suppression in the most recent year, implying that these individuals may be at risk for transmitting HIV to others [30]. Moreover, in 2011, the mean CVL in DC, was 31,393 copies/ml, indicative of a high rate of potential onward transmissions and a failure of the HIV care continuum at one or more stages [31]. DC surveillance data have indicated that seven of the city’s eight geopolitical areas, or Wards, have an HIV prevalence greater than 1% [23] and when mapped, the distribution of CVL in DC was highest in geographic areas of higher poverty and lowest educational attainment [16].
Thus while significant progress has been made in curbing the HIV epidemic in Washington, DC, these findings emphasize the need for an intensive focus on HIV diagnosis and treatment activities among the most high-risk populations. The objectives of this study were to assess the feasibility and relative utility of using routinely collected HIV surveillance data to identify geographic areas with the potential for high HIV transmission so that these areas could then be targeted for community-based HIV testing. Specifically, based on population-based surveillance data, inclusive of monitored viral load (mVL), a measure related to but distinct from CVL, we sought to identify and conduct testing in areas where we would be more likely to identify HIV-infected persons either unaware of their infection or needing linkage or re-engagement in care. We hypothesized that within high prevalence census tracts, the additional information provided by indicators reflecting the care continuum would enable a more efficient strategy for finding undiagnosed or untreated people living with HIV. We expected that community-based testing would identify more HIV-positive persons in census tracts with poorer care-continuum outcomes and higher mVL compared to those tracts with better care-continuum outcomes and lower mVL.
Methods
Data source and continuum of care indicators
We used the most current data available from the DC Department of Health HIV/AIDS, Hepatitis, STD, TB Administration enhanced HIV/AIDS Surveillance Reporting System (eHARS) and routinely reported laboratory data to identify all cases of HIV diagnosed from 2008 through 2012, ages 13 years or older at the time of diagnosis, and alive in 2013. Individuals who were not DC residents, homeless, incarcerated, or without a known street address (missing data or post office box address) were excluded from the analysis. eHARS surveillance and laboratory data were used to calculate three HIV care-continuum indicators for each census tract: monitored viral load (mVL), the proportion of persons diagnosed but never in care (NIC), and the proportion of persons out of care (OOC). Monitored viral load (mVL) is one approach used to measure the level of circulating virus in a population and the potential for transmission. mVL is measured through the assessment of laboratory data (e.g., HIV RNA) from routinely collected HIV surveillance and laboratory systems. Defined as the geometric mean of the test results of those persons who have been linked to care and have a subsequent reported viral load test, the mVL provides a rough indication of access and adherence to ART among patients captured by surveillance systems [15]. The proportion of persons NIC was measured by calculating the proportion of individuals diagnosed with HIV but with no subsequent CD4 or viral load measures indicative of linkage to care. Finally, the proportion of persons out of care was defined as the proportion of persons diagnosed with HIV who did not have any reported lab results in 2013, inclusive of those who were never in care. A statistical algorithm was then developed to stratify the census tracts into two groups: those above the median for all 3 indicators, or “higher risk areas” and those below the median for all three indicators, or “lower risk areas”.
Selection of census tracts for HIV testing
We calculated the number of cases in each of the 179 Washington, DC census tracts, excluding those tracts with fewer than five cases. Then, using eHARS data, we estimated the percent of the population diagnosed with HIV per 100,000 population (referred to as ‘prevalence’) for the remaining census tracts (N=160) by dividing the number of cases by the most recent available (2011) population count of individuals 13 years and older, and dividing by 100,000. Census tracts with prevalence greater than 1% were selected as the pool of potential targets for inclusion in testing efforts (N=55 tracts) in keeping with the UNAIDS definition of a generalized epidemic threshold of 1% [32]. Within these tracts with high prevalence, we defined “higher risk tracts” (HRAs) as those that were equal to or above the median of the 55 tracts on CVL (expressed as a geometric mean: 143.78 copies/ml), proportion out of care (36.7%), and proportion never in care (11.4%). Similarly, “lower risk tracts” (LRAs) were defined as those below the median on all three indicators. These criteria resulted in the identification of 12 HRAs and 8 LRAs, which were selected for HIV testing. To examine group differences between the higher and lower risk groups, we used data from the 2012 American Community Survey [33] to obtain socioeconomic characteristics of the selected tracts. Due to the small number of tracts per group, effect sizes were calculated to identify potential differences between the groups [34]. The twenty census tracts and their related continuum of care outcomes were mapped using ArcGIS and used to identify the areas for targeted HIV testing.
Participants and procedures for HIV testing and survey completion
Community-based HIV testing was conducted by Community Education Group (CEG), a local community based organization, using a mobile unit that rotated through the selected tracts. Founded in 1993, CEG is a DC community-based nonprofit organization that uses intensive outreach and navigators to conduct HIV testing and increase linkage and retention in care among marginalized populations [35]. CEG community health workers indigenous to the target communities conducted HIV testing in each of the 20 census tracts with a goal of conducting 75 tests in each of the 20 tracts. The CEG mobile unit was parked in multiple locations throughout the census tract and conducted testing either throughout a two-week period or until the goal number of tests was performed. Once testing had occurred at each census tract, a second round of testing was conducted to attempt to achieve the testing targets.
Eligible participants were identified using street outreach, were 18 years of age or older, and self-reported HIV negative. Participants did not have to reside in the census tract where testing was taking place. Persons who had been tested by CEG within the previous 90 days or who self-identified as HIV positive were excluded from participation. After providing verbal consent, participants were asked to complete a brief survey regarding their demographics, risk behaviors, the acceptability of certain HIV prevention interventions, and place of residence. All survey data were entered using touch screen tablets into the Health Information Virtual Exchange (HIVE) system, a CEG HIPAA-compliant individual patient health record system. Confidential rapid HIV testing was performed using Oraquick rapid HIV tests. Participants received a $10 gift card for HIV testing, and those who tested positive received up to $50 in gift cards for immediate linkage to care and transportation to a clinic site for confirmatory testing by CEG staff.
Analyses and IRB
Participant survey data were extracted from HIVE for analysis and residential addresses were converted to census tracts. We performed descriptive and univariate analyses using SAS version 9.3 (Cary, NC), and mapping using ArcGIS to compare testing results and individual level-risk behaviors in the two areas. All study materials and protocols were approved by the George Washington University and the District of Columbia Department of Health Institutional Review Boards.
Results
Geographic distribution of census tracts for testing
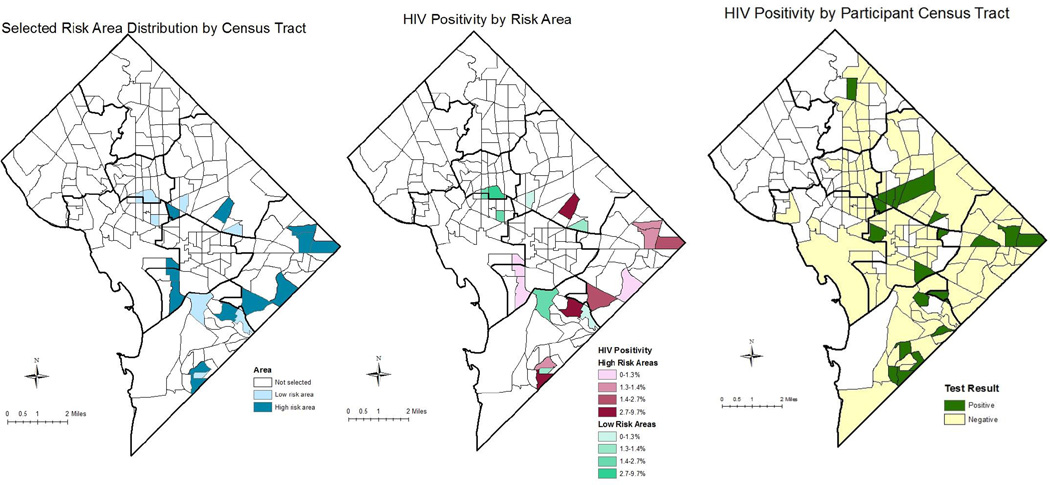
The map in Figure 1a demonstrates the geographic distribution of the selected census tracts based on meeting the criteria of being an HRA or LRA. The selected census tracts represented areas in six of the city’s eight geopolitical regions or Wards. At the census tract level, among the twelve HRAs, the HIV prevalence ranged from 1.02 to 1.84 cases per 100 population, the mVL ranged from 151.8 to 488.5 copies/ml, the highest proportion of persons never in care and out of care were 29.0% and 48.6%, respectively, and the highest proportion virally suppressed was 50% (Table 1). In contrast, among the eight LRAs, HIV prevalence ranged from 1.0 to 1.91 cases per 100 population, the mVL ranged from 26.5 to 138.3 copies/ml, and the highest proportion of persons never in care and out of care were 10.6% and 35.7%, respectively. The highest proportion virally suppressed was 62.5%.
Figure 1. Geographic distribution of risk areas, and HIV positivity by risk area and participant census tract.
This figure shows the geographic distribution of the higher (HRA, n=12) and lower (LRA, n=8) risk areas where testing was conducted and the HIV positive tests by risk group area and participant residential census tract. Map A shows the distribution of the 20 census tracts selected for testing based on the statistical algorithm. Map B shows the distribution of the proportion of HIV positive tests by census tract of testing. Map C shows the distribution of HIV positive tests by the participant’s residential census tract. “Positive” implies that at least one positive HIV test was identified among a participant living in that census tract. For all maps, the darker black lines represent the borders of the 8 geopolitical designations or Wards in Washington, DC. One or more participants tested HIV positive in 16 of the 20 census tracts selected for testing. Positivity ranged from 0% to 9.7%. Participants resided throughout the city; however, most of those testing positive lived in a Ward that had been selected for testing.
Table 1.
HIV Continuum of Care Indicators by Census Tract Risk Area Designation, N=20
| Risk Area Designation |
Population (≥13 yrs) |
HIV Prevalence (per 100 population)* |
Monitored Care VL (geometric mean) (copies/ml) |
% Never in Care |
% Out of Care |
% Virally suppressed* |
|---|---|---|---|---|---|---|
| Higher | 1,257 | 1.03 | 277.2 | 23.1 | 46.1 | 30.8 |
| Higher | 1,728 | 1.45 | 246.7 | 16.0 | 40.0 | 40.0 |
| Higher | 1,832 | 1.69 | 151.8 | 29.0 | 45.1 | 33.3 |
| Higher | 2,062 | 1.84 | 155.8 | 21.1 | 36.8 | 36.8 |
| Higher | 2,394 | 1.25 | 246.3 | 13.3 | 36.7 | 40.0 |
| Higher | 2,504 | 1.28 | 432.3 | 12.5 | 40.6 | 31.3 |
| Higher | 2,692 | 1.30 | 168.9 | 13.9 | 38.9 | 50.0 |
| Higher | 2,743 | 1.28 | 167.7 | 11.4 | 40.0 | 45.7 |
| Higher | 2,942 | 1.02 | 333.9 | 16.7 | 43.3 | 37.9 |
| Higher | 3,112 | 1.19 | 202.6 | 18.9 | 48.6 | 35.1 |
| Higher | 3,157 | 1.24 | 488.5 | 12.8 | 46.2 | 28.9 |
| Higher | 3,315 | 1.48 | 269.9 | 14.3 | 36.7 | 38.8 |
| Lower | 1,288 | 1.24 | 85.2 | 0 | 6.3 | 62.5 |
| Lower | 1,784 | 1.91 | 81.8 | 5.9 | 26.5 | 55.9 |
| Lower | 2,099 | 1.00 | 73.3 | 4.8 | 28.6 | 60.0 |
| Lower | 2,425 | 1.15 | 38.0 | 3.6 | 21.4 | 57.1 |
| Lower | 2,826 | 1.73 | 69.8 | 8.2 | 32.7 | 57.1 |
| Lower | 3,048 | 1.08 | 29.4 | 5.9 | 29.4 | 55.9 |
| Lower | 3,638 | 1.15 | 138.3 | 7.1 | 35.7 | 42.5 |
| Lower | 4,321 | 1.06 | 26.5 | 10.6 | 34.0 | 57.4 |
HIV prevalence and proportion virally suppressed were not included in the determination of risk area designation but are included here for informational purposes.
Comparison of sociodemographic and risk area characteristics
Table 2 shows the characteristics of the selected census tracts by group. As expected based on the selection criteria, the groups differed greatly by mVL, percent out of care, and percent never in care, with effect sizes (Cohen’s d) all greater than 2.0. The HRAs had substantially lower per capita income, lower percent of the population with a bachelor’s degree, and a higher percent of the population that was Black compared to the LRAs. In contrast, the percent of the population living below poverty level was similar in the two groups.
Table 2.
Descriptive statistics for the higher and lower risk groups identified within tracts with HIV prevalence ≥ 1 percent
| size | Higher Risk Areas (N=12) | Lower Risk Tracts (N=8) | Effect |
|---|---|---|---|
| Surveillance Data | Mean (S.D.) | Mean (S.D.) | Cohen’s d |
| HIV prevalence (per 100) | 1.337 (0.2439) | 1.292 (0.3365) | 0.15 |
| Monitored CVL | 261.8 (109.0) | 67.8 (37.0) | 2.39 |
| Percent out of care | 42.6 (4.2) | 26.8 (9.5) | 2.02 |
| Percent never in care | 16.9 (5.2) | 5.8 (3.2) | 2.58 |
| Per capita income (US$) | 23,633 (12,222) | 35,040 (24,174) | 0.60 |
| Percept below poverty level | 29.7 (14.1) | 28.9 (19.3) | 0.05 |
| Percent Black | 88.0 (16.9) | 68.3 (34.4) | 0.72 |
| Percent with education of B.A. or higher |
21.9 (15.9) | 35.7 (29.2) | 0.59 |
| Population | 2,478 (642) | 2,679 (993) | 0.24 |
Note: Cohen’s d effect sizes are considered large at .8, medium at .5, and small at .2 (Cohen, 1992).
Distribution and characteristics of HIV testing participants
Between mid-July 2015 and mid-October 2015, a total of 1,471 participants were tested and surveyed: 870 from the HRAs and 601 from the LRAs. The number of participants tested per census tract ranged from 63 to 87, with a mean of 73.6. Table 3 shows the characteristics of the participants recruited from the HRAs and LRAs. Groups were similar in age, education, and proportion of participants who were injection drug users or who reported male-to-male sexual activity. However, the groups differed in gender and racial distribution, with a higher proportion of males tested in the LRAs (63.7% vs. 56.7%, p=0.0067) and a greater representation of Black participants in the higher risk group (94.6% vs. 91.0%, p=0.0076). Participants also differed with respect to residence in the census tract in which they were tested, with 18.5% of those testing in the LRAs residing in the tract compared to 25.5% of those in the HRAs (p=0.0015). No significant differences were observed with respect to risk behaviors when comparing the two testing groups.
Table 3.
Demographic, risk behavior, and HIV testing results for participants sampled from the higher and lower risk areas (N=1,471)
| Total N=1,471 |
HRA N=870 |
LRA N=601 |
Testing HIV positive N=28 |
|||
|---|---|---|---|---|---|---|
| Characteristic | N (%) | N (%) | N (%) | N (%) | p-value1 | |
| Age (median, range) | 47 (18–81) | 47 (18–81) | 48 (18–80) | 50.5 (29–59) | 0.84 | |
| Male gender | 876 (59.6) | 493 (56.7) | 383 (63.7) | 18 (64.3) | 0.0067 | |
| Black | 1,370 (93.1) | 823 (94.6) | 547 (91.0) | 28 (100) | 0.0076 | |
| Less than high School education |
470 (32.0) | 286 (32.9) | 184 (30.6) | 5 (17.9) | 0.36 | |
| Uninsured | 132 (9.0) | 86 (9.9) | 46 (7.7) | 2 (7.1) | 0.14 | |
| Residence in testing area |
333 (22.6) | 222 (25.5) | 111 (18.5) | 2 (7.1) | 0.0015 | |
| Injection drug use (in prior 12 months) |
64 (4.4) | 31 (3.6) | 33 (5.6) | 2 (7.1) | 0.073 | |
| Men who have sex with men |
35 (2.4) | 20 (2.3) | 15 (2.5) | 4 (14.3) | 0.81 | |
| Heterosexual sex | 909 (61.8) | 534 (61.4) | 375 (62.4) | 16 (57.1) | 0.69 | |
| Number of main sex partners (median, range) |
1 (0–12) | 1 (0–12) | 1 (0–7) | 0 (0–2) | 0.91 | |
| Had at least one main sex partner |
875 (59.5) | 513 (59.0) | 362 (60.2) | 13 (46.4) | 0.63 | |
| Know main partner HIV status (always) (n=875)2 |
516 (59.0) | 306 (59.7) | 210 (58.0) | 6 (46.2) | 0.63 | |
| Condom with main partner (always) (n=875) |
214 (24.5) | 124 (24.2) | 90 (24.9) | 5 (38.5) | 0.82 | |
| Main partner HIV negative (n=741) |
683 (92.2) | 383 (91.2) | 300 (93.5) | 10 (76.9) | 0.26 | |
| Number of casual sex partners (median, range) |
0 (0–54) | 0 (0–54) | 0 (0–30) | 0 (0–5) | 0.71 | |
| Had at least one casual sex partner |
300 (20.4) | 175 (20.1) | 125 (20.8) | 6 (21.4) | 0.75 | |
| Know casual partner status (always) (n=300) |
86 (28.7) | 51 (29.1) | 35 (28.0) | 0 (0) | 0.83 | |
| Condom with casual partner (always) (n=300)3 |
120 (40.0) | 76 (43.4) | 44 (35.2) | 1 (16.7) | 0.15 | |
| HIV Testing Results | ||||||
| Number of tests completed (mean, range) |
73.6 (63–87) | 72.5 (70–75) | 75.1 (63–87) | -- | -- | |
| Number of reactive tests (mean, range) |
1.4 (0–7) | 1.5 (0–7) | 1.3 (0–2) | -- | -- | |
| Test positivity (mean, range) |
1.9 (0–9.7) | 2.1 (0–9.7) | 1.7 (0–2.8) | -- | -- | |
| Number of participants testing HIV positive |
28 (1.9) | 18 (2.1) | 10 (1.7) | -- | 0.57 | |
| Newly diagnosed | 9 (32.1) | 5 (27.8) | 4 (40.0) | -- | -- | |
|
Previously diagnosed and in care |
13 (46.4) | 8 (44.4) | 5 (50.0) | -- | -- | |
|
Previously diagnosed and out of care |
6 (21.4) | 5 (27.8) | 1 (10.0) | -- | -- | |
P-value comparing HRA and LRA distributions. P values ≤ 0.05 are bolded to indicate statistical significance.
n=13 for those testing HIV positive.
n=6 for those testing HIV positive.
HIV positivity and characteristics and care status of HIV positive participants
Among 1,471 persons tested, 28 (1.9%) participants had reactive results; 18 in the HRAs and 10 in the LRAs (Table 3). Six tests (0.4%) were invalid results, five of which occurred among participants in the HRA. Among the 28 persons testing HIV-positive, despite pre-screening, after receiving their HIV test results, 19 people stated they were previously known to be HIV positive of which 13 were already in care and six reported being out of care. Nine new HIV diagnoses were identified, of which five were identified in an HRA. Thus out of 28 HIV positive tests, 15 (54%) were new diagnoses or out of care.
Demographically, the median age among persons screening HIV positive was 50.5 years, all were Black, 64% were male, 7% were uninsured and 18% had less than a high school education. Self-reported risk behaviors among those testing positive found that 14% reported engaging in male-to-male sex, 57% in heterosexual sex, and 7% reported injection drug use in the prior 12 months. Among those reporting at least one main sexual partner, 46% reported always knowing their main partner’s HIV status, 77% reported that their main partner was HIV negative, and 39% reported always using a condom with their main partner. Among those reporting at least one casual partner, no participants reported always knowing their casual partner’s HIV status, and only one person reported always using a condom with their casual partners (Table 3).
Geographic distribution of positive tests
Positivity in the HRA was 2.1% and ranged from 0 to 9.7%; compared to 1.7% in the lower risk area where positivity rates ranged from 0 to 2.8% (Table 3). Although the positivity rate was higher in the HRA, the difference in rates between the two areas was not statistically significant (p=0.57). In the tract with 9.7% of participants testing positive, five of the seven persons testing positive were tested on the same day with a total of 18 tests conducted in that tract on that particular day. In addition, all seven participants reported being previously diagnosed with HIV, with two being out of care. The geographic distribution of those testing HIV-positive by risk area and by participant residence is shown in Figure 1B and 1C. When looking at the geographic distribution of HIV testing results by risk area, 16 of the 20 tracts identified at least one HIV positive person, with the highest proportions of persons testing positive being found in two of the HRAs. Additionally, four CTs in the twelve HRAs were in the top quartile for positivity; two of the CTs in the eight LRAs were in the top quartile for positivity (Figure 1B). Residential CT data was available for 91% of participants. Distribution of the participants’ CTs found that participants lived in 170 CTs, of which 124 tracts were in DC and encompassed seven of the city’s eight Wards (Figure 1C). Twenty-three percent of participants tested lived in the same CT in which their test occurred. Among the 28 participants testing positive, residential CTs were available for 25 participants. HIV positive participants lived in 20 different CTs with five HIV positive participants living in one of the targeted CTs, and only two residing in the CT in which they were tested.
Discussion
We hypothesized that surveillance data could be used to create an efficient strategy for targeting geographic hot spots for community-based testing and that by stratifying areas by risk, we would identify a larger number of HIV-positive persons who were unaware of their diagnoses or needed re-linkage to care in higher risk areas. We also expected that conceptualizing HIV risk by accounting for not only mVL, but also the percent of HIV-positive individuals out of care or never in care, would be especially effective. Although our findings provided some evidence of the feasibility and utility of using surveillance data to identify geographic areas for targeted HIV testing, we did not find strong support that our approach identified differences in the targeted testing areas.
The use of community-based testing in the selected areas proved feasible. By working with CEG, a local community based organization adept at identifying and encouraging HIV testing among hard-to-reach populations, using a field-based approach we were able to test almost 1,500 individuals in a three-month period and effectively link or relink those testing positive to care. Evidence shows that community-level outreach workers, such as CEG’s, are effective at this type of outreach as they are culturally sensitive to the target populations, familiar with social networks, and well-equipped to engage HIV+ people into care [36–38]. Our identification of nine new diagnoses through community-based testing highlights the need for combination approaches to HIV testing, as the populations that may be at highest risk for HIV may not seek care in traditional clinical settings [12, 39, 40].
HIV testing was not only feasible but also yielded an overall HIV positivity of 1.9%. This positivity was slightly lower than the city-wide HIV prevalence (2.5%) in 2013 [41], but consistent with local positivity rates for routine, targeted, and emergency department testing of 0.44–1.7%, 1.3%, and 1.1%, respectively [25, 39, 42]. While we had anticipated identifying a high proportion of positive persons, our positivity was likely underestimated as we did not test persons who self-reported as positive during the recruitment and screening process. This was somewhat counteracted by those persons who tested but whom later self-identified as previously diagnosed. Nevertheless, community, field-based testing identified at least one HIV positive person in 80% of the targeted census tracts. Furthermore, in one area the positivity rate was 9.7%. While this high rate may have been due to sampling bias secondary to social network referral and word of mouth regarding the incentives, it could imply that we identified a local pocket of infection where focused prevention intervention efforts could be implemented in the context of a generalized epidemic setting [43, 44].
While use of surveillance data and HIV testing were feasible, we did not observe a statistical difference in HIV positivity between the two risk groups as defined by the care-continuum variables. Although all the targeted census tracts had prevalence rates above one percent, we had expected that testing would identify more HIV infections in areas characterized by higher mVL and higher proportions of people who were out of care or never in care than in those with lower levels. Potential reasons for the lack of difference may include not only the restriction of census tracts to those with a prevalence greater than one percent, but also the fact that we did not take prevalence into account in creating our high and low risk groupings. For example, it would be reasonable to expect a census tract with a prevalence of 1.9 might yield a greater proportion of newly identified cases than a census tract with a prevalence of 1.0, regardless of the continuum of care characteristics. In fact, we did not observe that targeted tracts with the highest prevalence based on surveillance data consistently yielded the highest positivity rates.
There are other aspects of our algorithm for forming the high and low risk groups that could have lessened the effectiveness of our approach to including the care-continuum variables. Rather than median splits, more extreme criteria would have resulted in groups that were more distinct. Because of the limited number of tracts with high prevalence and the inclusion of three care continuum variables, we were unable to obtain a sufficient sample of tracts with a stricter criterion, such as inclusion in the highest and lowest quartiles. This approach would be possible in a larger study with either a greater number of census tracts or by using a larger geographic unit, coupled with the ability to test more people for longer periods of time. Finally, our algorithm gave the three continuum of care characteristics equal weight; i.e., a tract had to be above or below the median on all three. Future research could assess the impact of these different indicators of care to determine the importance they should be given in guiding geographic areas for testing. Other revisions of the algorithm could incorporate routine HIV testing data, additional surveillance data such as sexually transmitted infections, rates of viral suppression, areas with extremely high viral loads (e.g., >100,000 copies/ml), and include global and local hot spot analyses [9, 11, 20].
Sampling issues may have also contributed to the failure to find a difference between higher and lower risk groups based on the continuum of care. Those tested represented a convenience sample of individuals who self-selected to get tested at the mobile unit, generally during weekday hours. Moreover, individuals who self-identified as HIV-positive during preliminary screening were not tested. While we do not have a record of how many individuals that included, they may have differed by risk group area. Further revisions to this testing approach could therefore include testing during evenings and weekends, confirming self-reported HIV status using surveillance data, and documenting the numbers of persons who were ineligible due to being HIV positive. In general, the effectiveness of this approach could also be measured by comparing it to other testing schemes such as using a multi-level approach which combines tract-level and individual-level data, venue-based, or clinic-based routine HIV testing [9, 11, 12, 40, 45–48], as these approaches may also successfully identify persons at high risk for HIV and have a higher yield of positives.
Limitations of our analysis include the small sample size of persons both tested and identified as HIV positive and thus the inability to control for potential confounders, as well as the choice of locations and times for testing. Obtaining reliable estimates of positivity in a sample of 75 people is difficult; thus an alternate approach might include testing more individuals through a demographic health survey or through door-to-door testing in order to achieve a more reliable estimate for a given geographic area [49–51]. Finally, while we relied on the residence at diagnosis from surveillance data to identify census tracts, most persons tested did not reside in the census tracts where their testing occurred, which may indicate that they are also engaging in risk behaviors elsewhere. This is a limitation of surveillance data as it is not able to capture the location where risk behaviors are occurring, hence future adaptations of this approach might consider inclusion of residents of the geographic unit of interest only.
In conclusion, our hypothesis that community-based testing would identify more HIV-positive persons in census tracts with poorer care-continuum outcomes and higher mVL compared to those with better care-continuum outcomes and lower mVL was not confirmed. However, we were able to demonstrate that partnering with a community based organization allowed for efficiency in testing large numbers of people and importantly helped to identify persons who were progressing suboptimally along the HIV care continuum resulting in identification of new diagnoses and persons who were out of care. Given the widespread availability of surveillance data at the local level, efforts to improve its accuracy, and refine local measurements of the HIV care continuum, other health departments conducting HIV surveillance could relatively easily adapt our approach to their local context and local geographic divisions to determine how best to target areas for HIV testing and other re-engagement efforts. Further research could refine this approach which incorporates the continuum of care and mVL and has the potential to result in more efficient strategies to achieve the NHAS goals of increasing the proportion of people tested, linked to care, and retained in care.
Acknowledgments
source of funding: This analysis was funded through a Center for AIDS Research supplemental grant through the District of Columbia Developmental Center for AIDS Research, an NIH-funded Program (5P30AI087714) and in support of the Public Health/Academic Partnership between the District of Columbia Department of Health, HIV/AIDS, Hepatitis, STD, TB Administration and The George Washington University School of Public Health and Health Services, Department of Epidemiology and Biostatistics (Contract Number POHC-2006-C-0030). All authors from the George Washington University, Community Education Group, as well as the District of Columbia Department of Health, reviewed and approved the final draft of the paper. Additionally, under the Partnership contract, the District of Columbia Department of Health had the right to review and approve the final version of the manuscript.
The authors would like to thank the staff at Community Education Group for their assistance with the recruitment, testing, and linkage to care of study participants; Dr. Marlene Smurzynski and Ms. Jenevieve Opoku for their assistance with compiling the HIV/AIDS surveillance data; and the study participants without whom these data would not be possible. The authors would also like to acknowledge the DC Center for AIDS Research (AI117970), an NIH funded program which is supported by the following NIH Co-Funding and Participating Institutes and Centers: NIAID, NCI, NICHD, NHLBI, NIDA, NIMH, NIA, FIC, NIGMS, NIDDK, and OAR. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to declare.
Portions of this paper were presented as an oral presentation at the 10th International Conference on HIV Treatment and Prevention Adherence (2015), Miami, FL. June 2015. “Feasibility of Using HIV Care Continuum Outcomes to Identify Geographic Areas for HIV Testing”, Abstract 216.
References
- 1.United States Office of National AIDS Policy. National HIV/AIDS Strategy: Federal Implementation Plan. 2010. White House Office of National AIDS Policy. 2010 Available at: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-2020-action-plan.pdf.
- 2.United States Office of National AIDS Policy. National HIV/AIDS Strategy for the United States: Updated to 2020. 2015 Available at: https://www.aids.gov/federal-resources/national-hiv-aids-strategy/nhas-update.pdf.
- 3.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: 2014. Available at: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en_0.pdf. [Google Scholar]
- 4.Skarbinski J, Rosenberg E, Paz-Bailey G, et al. Human immunodeficiency virus transmission at each step of the care continuum in the United States. JAMA Intern. Med. 2015;175(4):588–596. doi: 10.1001/jamainternmed.2014.8180. [DOI] [PubMed] [Google Scholar]
- 5.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20(10):1447–1450. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 6.Goswami ND, Schmitz MM, Sanchez T, et al. Understanding Local Spatial Variation Along the Care Continuum: The Potential Impact of Transportation Vulnerability on HIV Linkage to Care and Viral Suppression in High-Poverty Areas, Atlanta, Georgia. J Acquir Immune Defic Syndr. 2016;72(1):65–72. doi: 10.1097/QAI.0000000000000914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wand H, Ramjee G. Targeting the hotspots: investigating spatial and demographic variations in HIV infection in small communities in South Africa. J Int AIDS. Soc. 2010;13:41. doi: 10.1186/1758-2652-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Westercamp N, Moses S, Agot K, et al. Spatial distribution and cluster analysis of sexual risk behaviors reported by young men in Kisumu, Kenya. Int J Health Geogr. 2010;9:24. doi: 10.1186/1476-072X-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramjee G, Wand H. Geographical clustering of high risk sexual behaviors in"hot-spots" for HIV and sexually transmitted infections in Kwazulu-Natal, South Africa. AIDS Behav. 2014;18(2):317–322. doi: 10.1007/s10461-013-0578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shepard CW, Gortakowski HW, Nasrallah H, et al. Using GIS-Based Density Maps of HIV Surveillance Data to Identify Previously Unrecognized Geographic Foci of HIV Burden in an Urban Epidemic. Public Health Rep. 2011;126(5):741–749. doi: 10.1177/003335491112600517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goswami ND, Hecker EJ, Vickery C, et al. Geographic Information System-based Screening for TB, HIV, and Syphilis (GIS-THIS): A Cross-Sectional Study. In: Pai M, editor. PLoS ONE. 10. Vol. 7. 2012. p. e46029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gallagher KM, Sullivan PS, Lansky A, et al. Behavioral Surveillance Among People at Risk for HIV Infection in the U.S.: The National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(S1):32–38. doi: 10.1177/00333549071220S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eberhart MG, Yehia BR, Hillier A, et al. Behind the Cascade: Analyzing Spatial Patterns along the HIV Care Continuum. J Acquir Immune Defic Syndr (1999) 2013;64(0 1):S42–S51. doi: 10.1097/QAI.0b013e3182a90112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AIDSVu. Mapping the HIV Care Continuum. Available at: http://hivcontinuum.org/ [Google Scholar]
- 15.Centers for Disease Control and Prevention. Guidance on community viral load: a family of measures, definitions, and method for calculation. Available at: https://stacks.cdc.gov/view/cdc/28147.
- 16.Castel AD, Befus M, Willis S, et al. Use of the community viral load as a population-based biomarker of HIV burden. AIDS. 2012;26(3):345–353. doi: 10.1097/QAD.0b013e32834de5fe. [DOI] [PubMed] [Google Scholar]
- 17.Das M, Chu PL, Santos G-M, et al. Decreases in Community Viral Load Are Accompanied by Reductions in New HIV Infections in San Francisco. In: Carr JK, editor. PLoS ONE. 6. Vol. 5. 2010. p. e11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laraque F, Mavronicolas HA, Robertson MM, et al. Disparities in community viral load among HIV-infected persons in New York City. AIDS. 2013;27(13):2129–2139. doi: 10.1097/QAD.0b013e328360f619. [DOI] [PubMed] [Google Scholar]
- 19.Montaner JSG, Lima VD, Barrios R, et al. Expanded HAART Coverage is Associated with Decreased Population-level HIV-1-RNA and Annual New HIV Diagnoses in British Columbia, Canada. Lancet. 2010;376(9740):532–539. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Terzian AS, Bodach SD, Wiewel EW, et al. Novel Use of Surveillance Data to Detect HIV-Infected Persons with Sustained High Viral Load and Durable Virologic Suppression in New York City. In: Shoukry NH, editor. PLoS ONE. 1. Vol. 7. 2012. p. e29679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood E, Kerr T, Marshall B, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montaner JS, Wood E, Kerr T, et al. Expanded highly active antiretroviral therapy coverage among HIV-positive drug users to improve individual and public health outcomes. J Acquir Immune Defic Syndr. 2010;55(S1):S5–S9. doi: 10.1097/QAI.0b013e3181f9c1f0. [DOI] [PubMed] [Google Scholar]
- 23.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. District of Columbia HIV/AIDS, Hepatitis, STD, and TB Administration Epidemiology and Surveillance Report: Surveillance Data Through December 2012. District of Columbia Department of Health. 2013 Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/2013%20Annual%20Report%20Final%20Edit.pdf.
- 24.Saafir-Callaway B, Lago L, Olejemeh C, et al. Re-engagement in Care Leads to Sustained Engagement and Viral Suppression, Abstract #854. Presented at: 2015 Conference on Retroviruses and Opportunistic Infections; 2015; Seattle, WA. [Google Scholar]
- 25.Castel AD, Magnus M, Peterson J, et al. Implementing a Novel Citywide Rapid HIV Testing Campaign in Washington, D.C.: Findings and Lessons Learned. Public Health Rep. 2012;127(4):422–431. doi: 10.1177/003335491212700410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallmark CJ, Skillicorn J, Giordano TP, et al. HIV Testing Implementation in Two Urban Cities: Practice, Policy, and Perceived Barriers. In: Eugenin EA, editor. PLoS ONE. 10. Vol. 9. 2014. p. e110010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. DC HIV Behavior Study Series: Injection Drug Use: IDUs and HIV Infection in DC. DC Department of Health HIV Behavioral Series. 2010 Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/IDU_Behavior_Study_2010__0.pdf.
- 28.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. DC HIV Behavior Study Series: Heterosexual Relationships and HIV in Washington, DC - DC HIV Behavior Study Series. 2010 Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/HET_BEH_STUDY.PDF.
- 29.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. DC HIV Behavior Study Series: MSM in DC: A Lifelong Commitment to Stay HIV Free. 2013 Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/MSM%20Behavioral%20Study%20Final.pdf.
- 30.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. District of Columbia Department of Health; 2015. HIV Care and Ryan White Care Dynamics HIV/AIDS, Hepatitis, STD, and TB Data through 2014. Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/page_content/attachments/HAHSTA%20HIV%20Care%20Dynamics%20supplement%20FINAL.pdf. [Google Scholar]
- 31.Castel AD. Treatment as Prevention in Washington, DC. Presented at: in 3rd International HIV Treatment as Prevention Workshop; 2013; Vancouver BC, Canada. [Google Scholar]
- 32.WHO Guidelines Approved by the Guidelines Review Committee. World Health Organization; 2013. Guidelines for Second Generation HIV Surveillance: An Update: Know Your Epidemic. Available at: http://apps.who.int/iris/bitstream/10665/85511/1/9789241505826_eng.pdf. [PubMed] [Google Scholar]
- 33.United States Census Bureau. ACS Data Tables on American FactFinder. Available at: http://www.census.gov/acs/www/data/data-tables-and-tools/american-factfinder/
- 34.Cohen J. A power primer. Psychol Bull. 1992;112(1):155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Community Education Group. Available at: http://communityeducationgroup.org/ [Google Scholar]
- 36.Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV AIDS (Auckl) 2013;5:1–17. doi: 10.2147/HIV.S28912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson MA, Mugavero MJ, Amico KR, et al. Guidelines for Improving Entry Into and Retention in Care and Antiretroviral Adherence for Persons With HIV: Evidence-Based Recommendations From an International Association of Physicians in AIDS Care Panel. Ann Intern Med. 2012;156(11):817–294. doi: 10.7326/0003-4819-156-11-201206050-00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craw JA, Gardner LI, Marks G, et al. Brief strengths-based case management promotes entry into HIV medical care: results of the antiretroviral treatment access study-II. J Acquir Immune Defic Syndr. 2008;47(5):597–606. doi: 10.1097/QAI.0b013e3181684c51. [DOI] [PubMed] [Google Scholar]
- 39.Castel AD, Choi S, Dor A, et al. In: Comparing Cost-Effectiveness of HIV Testing Strategies: Targeted and Routine Testing in Washington, DC. PLoS ONE. 10. Rosenberg ES, editor. Vol. 10. 2015. p. e0139605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassett IV, Regan S, Mbonambi H, et al. Finding HIV in Hard to Reach Populations: Mobile HIV Testing and Geospatial Mapping in Umlazi Township, Durban, South Africa. AIDS Behav. 2015;19(10):1888–1895. doi: 10.1007/s10461-015-1012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.District of Columbia Department of Health HIV/AIDS, Hepatitis, STD, TB Administration. District of Columbia HIV/AIDS, Hepatitis, STD, and TB Administration Epidemiology and Surveillance Report: Surveillance Data Through December 2013. 2014 Available at: http://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/HAHSTA%20Annual%20Report%202013_Final.pdf.
- 42.Czarnogorski M, Brown J, Lee V, et al. The Prevalence of Undiagnosed HIV Infection in Those Who Decline HIV Screening in an Urban Emergency Department. AIDS Res Treat. 2011;2011:879065. doi: 10.1155/2011/879065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tanser F, de Oliveira T, Maheu-Giroux M, et al. Concentrated HIV sub-epidemics in generalized epidemic settings. Curr Opin HIV AIDS. 2014;9(2):115–125. doi: 10.1097/COH.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tanser F, Bärnighausen T, Cooke GS, et al. Localized spatial clustering of HIV infections in a widely disseminated rural South African epidemic. Int J Epidemiol. 2009;38(4):1008–1016. doi: 10.1093/ije/dyp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nunn A, Towey C, Chan PA, et al. Routine HIV Screening in an Urban Community Health Center: Results from a Geographically Focused Implementation Science Program. Public Health Rep. 2016;131(S1):30–40. doi: 10.1177/00333549161310S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sharma M, Ying R, Tarr G, et al. A systematic review and meta-analysis of community and facility-based approaches to address gaps in HIV testing and linkage in sub-Saharan Africa. Nature. 2015;528(7580):S77–S85. doi: 10.1038/nature16044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burns DN, DeGruttola V, Pilcher CD, et al. Toward an Endgame: Finding and Engaging People Unaware of Their HIV-1 Infection in Treatment and Prevention. AIDS Res Hum Retroviruses. 2014;30(3):217–224. doi: 10.1089/aid.2013.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lipsitz M, Segura E, Castro J, et al. Bringing testing to the people - benefits of mobile unit HIV/syphilis testing in Lima, Peru, 2007–2009. Int J STD AIDS. 2014;25(5):325–331. doi: 10.1177/0956462413507443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nguyen TQ, Gwynn RC, Kellerman SE, et al. Population prevalence of reported and unreported HIV and related behaviors among the household adult population in New York City, 2004. AIDS. 2008;22(2):281–287. doi: 10.1097/QAD.0b013e3282f2ef58. [DOI] [PubMed] [Google Scholar]
- 50.Thorpe LE, Greene C, Freeman A, et al. Rationale, design and respondent characteristics of the 2013–2014 New York City Health and Nutrition Examination Survey (NYC HANES 2013–2014) Prev Med Rep. 2015;2:580–585. doi: 10.1016/j.pmedr.2015.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thorpe LE, Charon Gwynn R, Mandel-Ricci J, et al. Study Design and Participation Rates of the New York City Health and Nutrition Examination Survey, 2004. Prev Chronic Dis. 2006;3(3):A94. [PMC free article] [PubMed] [Google Scholar]