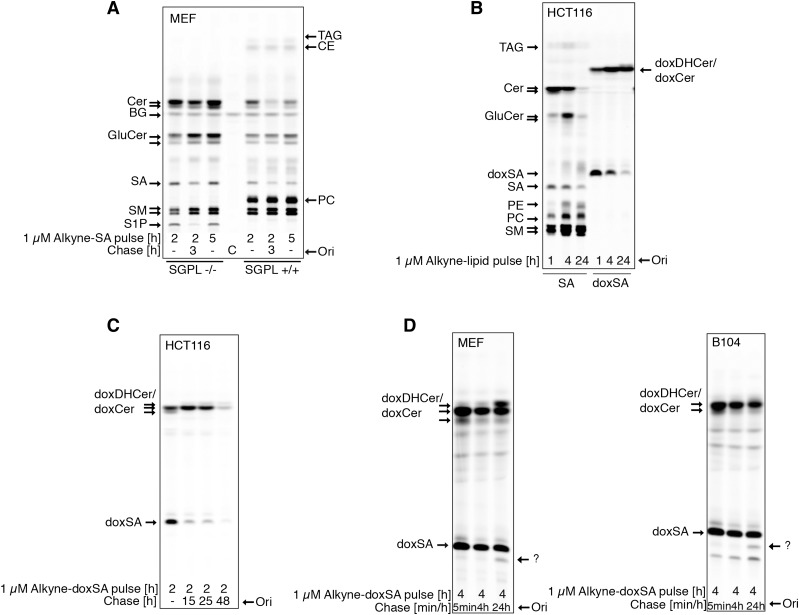
Fig. 2.
DeoxySLs are not recycled to canonical sphingolipids or fatty acids. Metabolic tracing of alkyne-doxSA and alkyne-SA by click fluorescence TLC. A: MEF WT or MEF S1P lyase−/− cells were treated with 1 μM alkyne-SA. Note that the alkyne signal is restricted to sphingolipids in MEF S1P lyase−/− cells, but also appears in glycerolipids in MEF WT cells. B. HCT116 cells were treated with 1 μM alkyne-SA or 1 μM alkyne-doxSA. Note that the alkyne signal of alkyne-doxSA was not detected in any canonical sphingolipids or glycerolipids. C: HCT116 cells were given a short pulse of 1 μM alkyne-doxSA for 2 h, followed by a chase up to 48 h in nonsupplemented growth medium. Note that the total alkyne signal decreased with prolonged chase times. D: MEF and B104 cells were treated with 1 μM alkyne-doxSA for 4 h, followed by a chase up to 24 h in nonsupplemented medium. The arrows indicate an alkyne signal that runs in a band at the height of SA after a 24 h chase, indicating a possible hydroxylated derivate of doxSA. Because of the lack of alkyne signal appearing in canonical sphingolipids or glycerolipids, it can be excluded that the hydroxylation would be at the C1 position. Please note that sphingolipid classes such as ceramides, glucosylceramides, and sphingomyelins run on the TLC according to the N-acyl fatty acid attached. Hence, most often, two bands (long-chain versus very-long-chain N-acyl metabolites) can be separated on the TLC. BG, background; C, control; CE, cholesterolester; Cer, ceramide; GluCer, glucosylceramide; Ori, origin; SM, sphingomyelin.