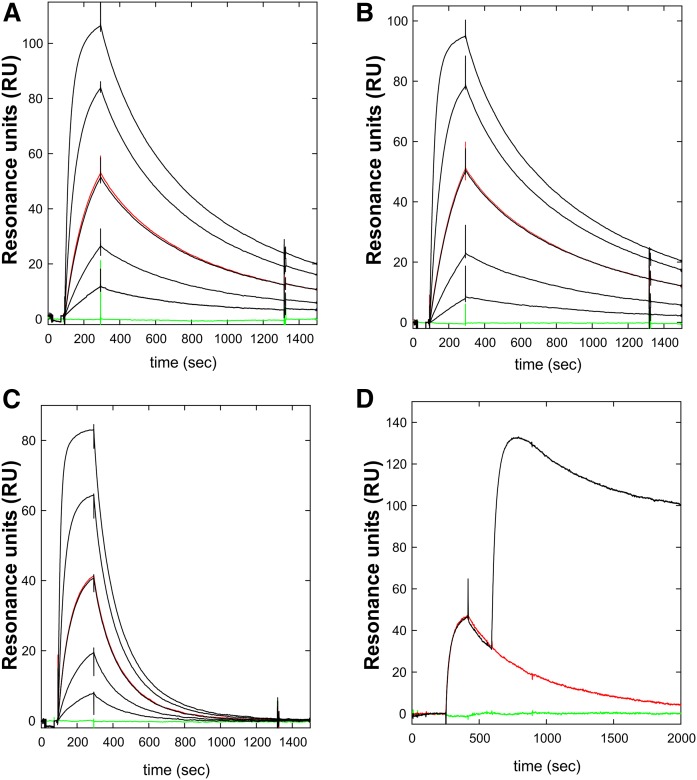
Fig. 3.
Kinetics for the interaction between mAb RE3 and GPIHBP1 by surface plasmon resonance. The real-time kinetic interactions between immobilized mAb RE3 and different GPIHBP1 proteins were measured with a BiacoreT200 system. A three-fold dilution series of wild-type GPIHBP1 (A); GPIHBP1-Δacidic (B); and GPIHBP1-W109S (C) were injected between 100 and 300 s, followed by a dissociation phase from 300 to 1,500 s. The concentrations analyzed were 90, 30, 10, 3, and 1 nM GPIHBP1 (black curves). One repeat measurement of 10 nM GPIHBP1 was performed at the end of each analysis (red); a buffer control curve is also shown (green). (D) Example of epitope binning. RE3 was immobilized on the sensor chip and 100 nM GPIHBP1 was captured by injection at 300 s, followed by a second injection of either buffer (red curve) or 100 nM mAb RF4 (black curve). The sensorgrams show that RE3 and RF4 belong to separate epitope bins and that their binding to GPIHBP1 was not mutually exclusive.