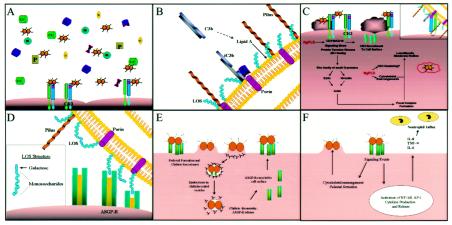
FIG. 1.
(A to C) Working model of gonococcal pathogenesis within the lower female genital tract. Alternative pathway complement components are produced and released by the cervical epithelia (A). Upon N. gonorrhoeae infection, complement is activated. Gonococcal pilus binds to the I-domain of CR3 allowing the bacterium to overcome the electrostatic repulsion between its own cell surface and that of the host cell. This places the gonococcus within proximity to the cervical cell surface where complement components would be sufficient to allow deposition upon the bacterial cell surface (B). C3b forms a covalent association with the lipid A core of gonococcal LOS and is rapidly inactivated to iC3b. The affinity of gonococcal porin for factor H may augment iC3b formation. The proximity of porin to LOS in the outer membrane may spatially favor the intimate association between iC3b and porin with the CR3 I-domain (C [inset]). Engagement of CR3 triggers a complex signal transduction cascade mediated by the Src-family tyrosine kinases and Rho GTPases (C). These processes result in vinculin- and ezrin-enriched focal complex formation and membrane ruffling, i.e., trigger mechanism of invasion. Secreted NgPLD modulates cervical cell signal transduction by playing a role in CR3 recruitment to the cervical cell surface and by modulating cytoskeletal reorganization. Additionally this protein augments intracellular survival of gonococci after their internalization within macropinosomes. Sialylated gonococci are eventually released from the cervical cell, where they are free to invade more of the cervical epithelia or where they become primed for transmission to the male urethra upon sialic acid removal by sialidases present within the female genital tract. (D) Invasion of the male urethral epithelium is mediated by an interaction between the terminal galactose moiety of LOS and the ASGP-R. (E) An intimate association occurs between the gonococcus and the urethral cell membrane, i.e., the zippering mechanism of invasion. Clathrin is recruited to the site of ASGP-R, and gonococcus-ASGP-R complexes are internalized in clathrin-coated pits in an actin-dependent process. Within the endosome a drop in pH is proposed to release the ASGP-R from the gonococcus surface, and clathrin molecules are lost. ASGP-R is recruited to the urethral cell surface, where it is available to serve as a receptor for more gonococci. (F) Engagement of the ASGP-R triggers cellular responses resulting in membrane pedestal formation beneath receptor-gonococcus complexes and activation of transcription factors required for the production of the inflammatory cytokines IL-6, IL-8, and TNF-α. Neutrophil influx accompanies cytokine release. Sialylated gonococci are eventually released from the urethral epithelium, where they can then be transmitted to a female partner. However, sialic acid on the gonococcus surface does not influence the ability of these organisms to associate with the uterine cervix.