Fig. 4. Deletion of spacer-1 enhances phosphorylation of several non-lysosomal glycoproteins.
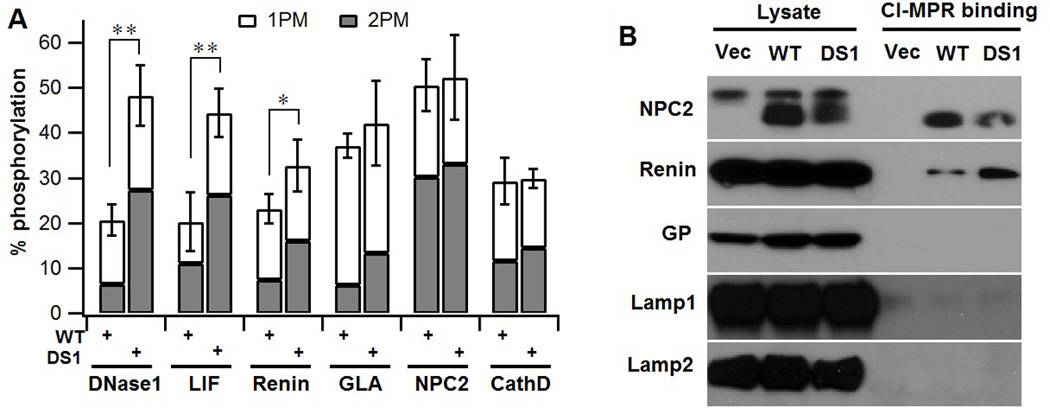
A. GNPTAB−/− HeLa cells were co-transfected with plasmids encoding either the 3 non-lysosomal proteins or 3 lysosomal proteins along with WT α/β precursor or the DS1 mutant cDNA. Cells were labeled with [2-3H]-mannose, followed by immunoprecipitation of the proteins secreted into the media and determination of the percent N-glycans containing either one (1PM) or two (2 PM) Man-6-P residues. In all instances, the lysosomal proteins secreted by the cells transfected with plasmids encoding only the lysosomal proteins contain less than 1 % phosphorylated glycans (10). N= 2–5. B. Western blot of GNPTAB−/− HeLa cells co-transfected with the expression plasmids for the indicated proteins along with empty vector, WT α/β precursor or the DS1 mutant cDNA. Cell lysates were incubated with CI-MPR-affinity beads and the binding of the various proteins was determined by probing the blot with the following antibodies: Renin - anti-HA; NPC2, GP, Lamp1 and Lamp2 with antibodies generated against the native protein.
